Now Available: Ultra-Sensitive ESR1 Mutation Liquid Biopsy Testing

Results are typically delivered within 7 days of sample receipt, enabling timely clinical decision making and study support.
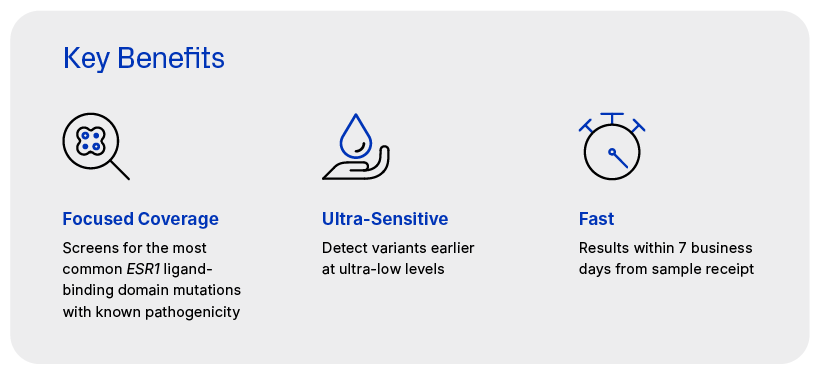
Our new ESR1 testing service provides ultra‑sensitive liquid biopsy detection of ESR1 mutations to support earlier, more informed treatment decisions.
To learn more on how this testing service can support your testing needs, please have your partner laboratory reach out to us at CLIAservices@bio-techne.com.
Why Test for ESR1?
The ESR1 gene encodes estrogen receptor alpha (ERα), which controls gene expression in response to estrogen. About 70% of breast cancers are hormone receptor–positive and depend on ERα signaling, making endocrine therapies—such as AIs, SERDs, and SERMs—standard treatment.
However, tumors can develop ESR1 mutations in the ligand‑binding domain, especially after prolonged aromatase inhibitor use. These mutations activate ERα even without estrogen, causing resistance to endocrine therapy and enabling disease progression. Several FDA‑approved treatments now specifically target ESR1-mutant disease detected via liquid biopsy.
Recent clinical trials (PADA‑1 and SERENA‑6) show that regularly monitoring patients for ESR1 mutations and switching therapy at first detection improves progression‑free survival. This suggests that early identification and intervention may prevent or delay treatment resistance.
Test Overview
| Technology | RT-qPCR |
| Coverage | E380Q, V422del, S463P, L536H/P/R (X), Y537S/N/C/D (X), D538G |
| Sample | 2 x 10mL Whole Blood (K2EDTA), spun down to plasma within 4 hours Plasma shipped frozen on dry ice |
| Assay Sensitivity | 0.03-0.11% VAF |
| Turnaround | 7 business days from receipt |
Test Limitations
This laboratory developed test consists of qualitative ESR1 mutation profiling It covers the following activating mutations: E380Q, V422del, S463P, L536H, L536P, L536R, Y537S, Y537N, Y537C, Y537D, D538G. ESR1 mutations outside of these regions will not be detected. The test cannot distinguish between mutations within a single codon. The presence of ESR1 mutations below LOD will not be reported.
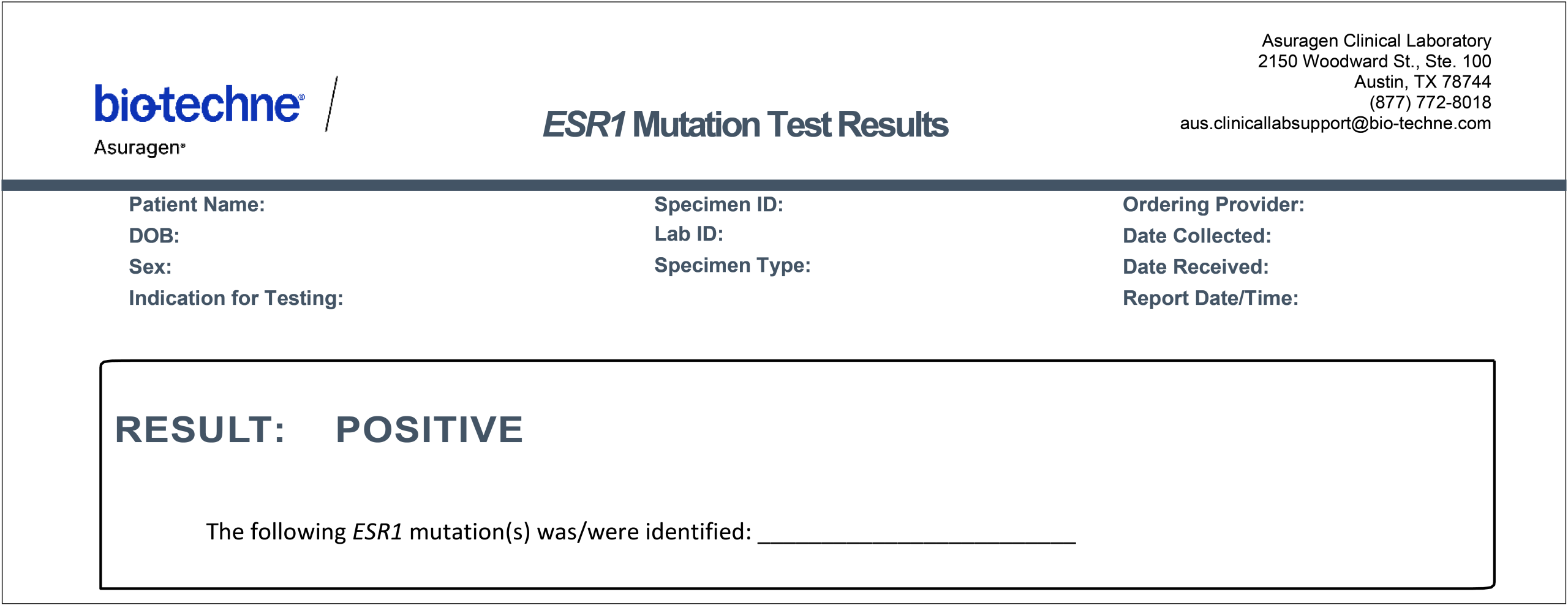
Sample Test Report
FAQs
How is this test billed?
Asuragen Clinical Laboratory will bill the ordering institution. We do not deal directly with payers.
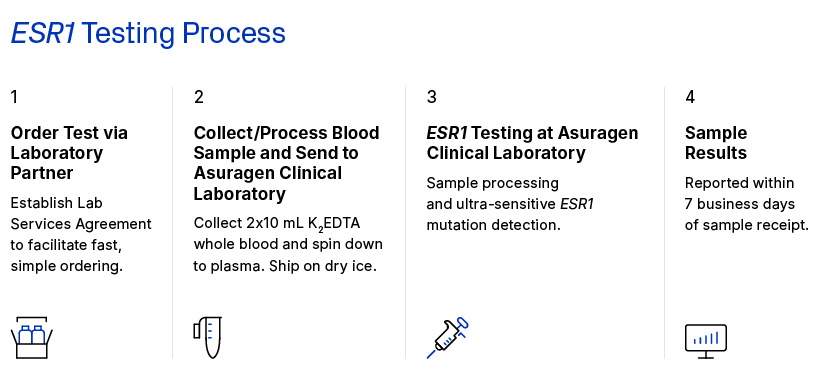
How do I order the test and where do I send samples?
Oncologists: contact your partner laboratory to request ESR1 testing through the Asuragen CLIA Laboratory.
Laboratories: Please contact your local Asuragen account manager for details or contact orders@asuragen.com to connect with your local representative.
What type of report will I receive, and how do I interpret the results?
Test reports will report whether any ESR1 mutation(s) were detected, which variant(s) were found, and the corresponding Cq values.
Which ESR1 mutations does this test detect?
The assay detects the 11 most common, pathogenic ligand-binding domain mutations in ESR1. The specific mutations include: E380Q, V422del, S463P, L536H*, L536P*, L536R*, Y537S**, Y537N**, Y537C**, Y537D**, D538G.
*Reported as L536X **Reported as Y537X
What sample types are accepted for testing?
2 x 10mL K2EDTA tubes of whole blood, spun down to plasma within 4 hours of venipuncture. Plasma should be shipped frozen overnight on dry ice to preserve sample integrity prior to testing.
How sensitive is the test for detecting ESR1 mutations?
Assay limit of detection varies by individual variant, but all are within the range of 0.03%-0.11%VAF
How quickly will I receive test results?
Results are delivered within 7 business days of sample receipt.
How can ESR1 mutation status guide treatment decisions?
ESR1 mutation status has implications for selecting targeted therapies in advanced, HR+/HER2- breast cancer and for clinical trial eligibility.1,2,3
Can this test be used to monitor treatment response over time?
Data is emerging demonstrating how ESR1 mutation surveillance can lead to downstream improvements in patient outcomes via early treatment intervention.4,5
Are there any known limitations of the test?
The assay is designed to exclusively detect 11 specific mutations within the ESR1 ligand-binding domain. Mutations outside of these select variants will not be detected or reported. Variants within a single location will be reported in aggregate (e.g., L536X). ESR1 copy number and percent variant allele frequency will not be reported. This test is validated only for use on plasma isolated from K2EDTA whole blood.
What quality controls are in place at the laboratory?
For a complete list of our licenses and accreditations, please visit https://asuragen.com/licenses-accreditations/.
Can this test detect ESR1 mutations in early-stage breast cancer?
This test is only validated for use with metastatic breast cancer patient samples.
Contact Us
Asuragen Clinical Laboratory
2150 Woodward St., Ste. 100
Austin, TX 78744
(877) 777-1874
CLIAservices@bio-techne.com
This test was developed and its performance characteristics determined by Asuragen Clinical Services. It has not been cleared or approved by the FDA. The laboratory is regulated under CLIA as qualified to perform high-complexity testing. This test is used for clinical purposes. It should not be regarded as investigational or for research.
- Brett JO et al. Breast Cancer Res 2021; 23:85
- Venetis K et al. Cancer Treatment Reviews 2023;121, 2023, 102642
- Bidard, FC et al. Journal of Clinical Oncology 2022; 40:28, 3246-3256
- Eveleigh D. et al. Presented at AMP 2025, Boston, USA https://asuragen.com/wp-content/uploads/2025/12/rt-qpcr-esr1-liquid-biopsy-test-validation.pdf
- Haynes BC. et al. Presented at ESMO Breast 2025, Berlin, Germany https://asuragen.com/wp-content/uploads/2025/05/esr1-mutation-monitoring-qpcr-assay-evaluation-esmo.pdf
Now Available: Ultra-Sensitive ESR1 Mutation Liquid Biopsy Testing

Results are typically delivered within 7 days of sample receipt, enabling timely clinical decision making and study support.
Our new ESR1 testing service provides ultra‑sensitive liquid biopsy detection of ESR1 mutations to support earlier, more informed treatment decisions.
To learn more on how this testing service can support your testing needs, please have your partner laboratory reach out to us at CLIAservices@bio-techne.com.
Why Test for ESR1?
The ESR1 gene encodes estrogen receptor alpha (ERα), which controls gene expression in response to estrogen. About 70% of breast cancers are hormone receptor–positive and depend on ERα signaling, making endocrine therapies—such as AIs, SERDs, and SERMs—standard treatment.
However, tumors can develop ESR1 mutations in the ligand‑binding domain, especially after prolonged aromatase inhibitor use. These mutations activate ERα even without estrogen, causing resistance to endocrine therapy and enabling disease progression. Several FDA‑approved treatments now specifically target ESR1-mutant disease detected via liquid biopsy.
Recent clinical trials (PADA‑1 and SERENA‑6) show that regularly monitoring patients for ESR1 mutations and switching therapy at first detection improves progression‑free survival. This suggests that early identification and intervention may prevent or delay treatment resistance.
Test Overview
| Technology | RT-qPCR |
| Coverage | E380Q, V422del, S463P, L536H/P/R (X), Y537S/N/C/D (X), D538G |
| Sample | 2 x 10mL Whole Blood (K2EDTA), spun down to plasma within 4 hours Plasma shipped frozen on dry ice |
| Assay Sensitivity | 0.03-0.11% VAF |
| Turnaround | 7 business days from receipt |
Test Limitations
This laboratory developed test consists of qualitative ESR1 mutation profiling It covers the following activating mutations: E380Q, V422del, S463P, L536H, L536P, L536R, Y537S, Y537N, Y537C, Y537D, D538G. ESR1 mutations outside of these regions will not be detected. The test cannot distinguish between mutations within a single codon. The presence of ESR1 mutations below LOD will not be reported.
Sample Test Report
FAQs
How is this test billed?
Asuragen Clinical Laboratory will bill the ordering institution. We do not deal directly with payers.
How do I order the test and where do I send samples?
Oncologists: contact your partner laboratory to request ESR1 testing through the Asuragen CLIA Laboratory.
Laboratories: Please contact your local Asuragen account manager for details or contact orders@asuragen.com to connect with your local representative.
What type of report will I receive, and how do I interpret the results?
Test reports will report whether any ESR1 mutation(s) were detected, which variant(s) were found, and the corresponding Cq values.
Which ESR1 mutations does this test detect?
The assay detects the 11 most common, pathogenic ligand-binding domain mutations in ESR1. The specific mutations include: E380Q, V422del, S463P, L536H*, L536P*, L536R*, Y537S**, Y537N**, Y537C**, Y537D**, D538G.
*Reported as L536X **Reported as Y537X
What sample types are accepted for testing?
2 x 10mL K2EDTA tubes of whole blood, spun down to plasma within 4 hours of venipuncture. Plasma should be shipped frozen overnight on dry ice to preserve sample integrity prior to testing.
How sensitive is the test for detecting ESR1 mutations?
Assay limit of detection varies by individual variant, but all are within the range of 0.03%-0.11%VAF
How quickly will I receive test results?
Results are delivered within 7 business days of sample receipt.
How can ESR1 mutation status guide treatment decisions?
ESR1 mutation status has implications for selecting targeted therapies in advanced, HR+/HER2- breast cancer and for clinical trial eligibility.1,2,3
Can this test be used to monitor treatment response over time?
Data is emerging demonstrating how ESR1 mutation surveillance can lead to downstream improvements in patient outcomes via early treatment intervention.4,5
Are there any known limitations of the test?
The assay is designed to exclusively detect 11 specific mutations within the ESR1 ligand-binding domain. Mutations outside of these select variants will not be detected or reported. Variants within a single location will be reported in aggregate (e.g., L536X). ESR1 copy number and percent variant allele frequency will not be reported. This test is validated only for use on plasma isolated from K2EDTA whole blood.
What quality controls are in place at the laboratory?
For a complete list of our licenses and accreditations, please visit https://asuragen.com/licenses-accreditations/.
Can this test detect ESR1 mutations in early-stage breast cancer?
This test is only validated for use with metastatic breast cancer patient samples.
Contact Us
Asuragen Clinical Laboratory
2150 Woodward St., Ste. 100
Austin, TX 78744
(877) 777-1874
CLIAservices@bio-techne.com
This test was developed and its performance characteristics determined by Asuragen Clinical Services. It has not been cleared or approved by the FDA. The laboratory is regulated under CLIA as qualified to perform high-complexity testing. This test is used for clinical purposes. It should not be regarded as investigational or for research.
- Brett JO et al. Breast Cancer Res 2021; 23:85
- Venetis K et al. Cancer Treatment Reviews 2023;121, 2023, 102642
- Bidard, FC et al. Journal of Clinical Oncology 2022; 40:28, 3246-3256
- Eveleigh D. et al. Presented at AMP 2025, Boston, USA https://asuragen.com/wp-content/uploads/2025/12/rt-qpcr-esr1-liquid-biopsy-test-validation.pdf
- Haynes BC. et al. Presented at ESMO Breast 2025, Berlin, Germany https://asuragen.com/wp-content/uploads/2025/05/esr1-mutation-monitoring-qpcr-assay-evaluation-esmo.pdf