AmplideX® PCR/CE SMN1/2 Plus Kit

Copy number variations in SMN1 and SMN2 are, respectively, associated with the onset and severity of spinal muscular atrophy (SMA), a debilitating and life-threatening illness of the central nervous system. Recent studies have demonstrated that transmission risk and disease severity may be impacted by the presence of additional variants, such as SMN1 gene duplication events and disease modifier in SMN2.
The AmplideX® PCR/CE SMN1/2 Plus Kit* revolutionizes the analysis of these two genes by delivering comprehensive results in less than four hours. Powered by AmplideX technology, the assay accurately quantifies SMN1 and SMN2 exon 7 copy number and also detects SMN1 gene duplication and SMN2 disease modifier variants – all from a single reaction. The assay shares a common workflow with other assays in the AmplideX product portfolio and is optimized for use on widely established laboratory equipment.
Order the AmplideX SMA Plus Kit
Features & Benefits
Reduced Complexity
- Similar workflow to AmplideX PCR/CE FMR1*† kit eases implementation and training
- Multiplexed, scalable design allows analysis of single-nucleotide variants, small indels, and copy-number changes from a single PCR reaction
- Assay-specific software automates variant calls and streamlines data analysis
Optimized Workflow
- DNA-to-data in less than four hours with only 60 minutes of hands-on-time
- Optimized for use on commonly installed CE equipment
- Fully-kitted solution sourced from a single vendor
Quality Results
- Ability to differentiate between 0, 1, 2, 3 and ≥4 copies for both SMN1 and SMN2
- Automated variant and copy-number genotyping
- Accuracy demonstrated through comparisons with multiple orthogonal methods
*For Research Use Only. Not for use in Diagnostic procedures.
Analytical Performance
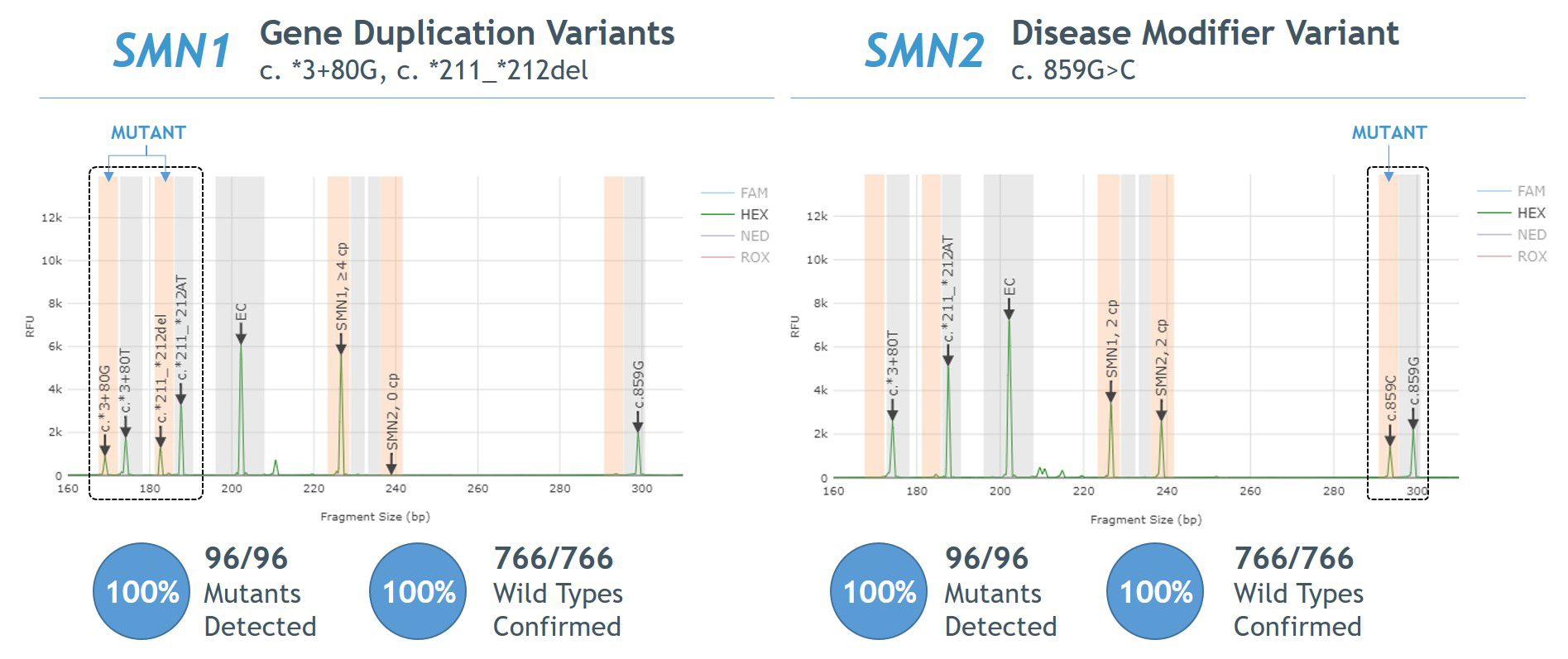
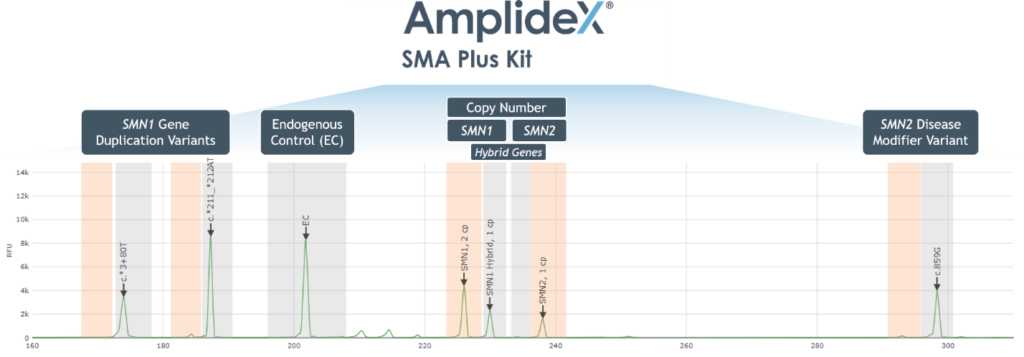
- Automated reporting of SMN1/2 copy number and variant detection via AmplideX PCR/CE SMN1/2 Plus Analysis Module (Figure 1)
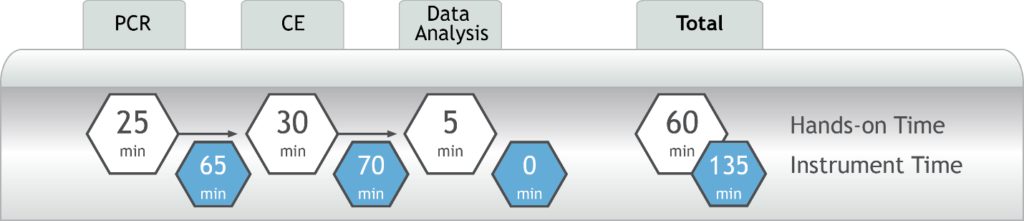
- DNA to data in less than four hours – all from a single PCR reaction (Figure 2)
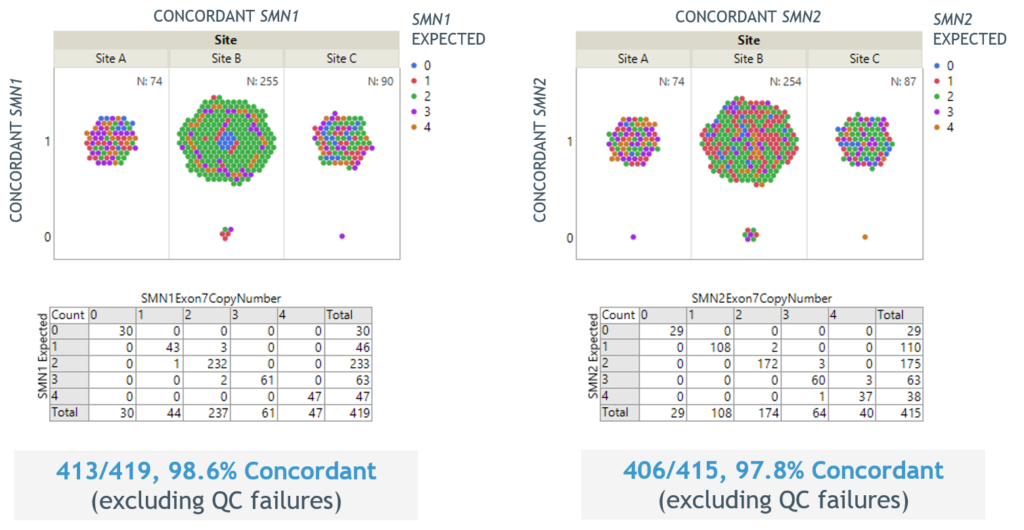
- Excellent concordance of SMN1 and SMN2 copy number results to sites’ in-house methods for over 500 replicates (Figure 3)
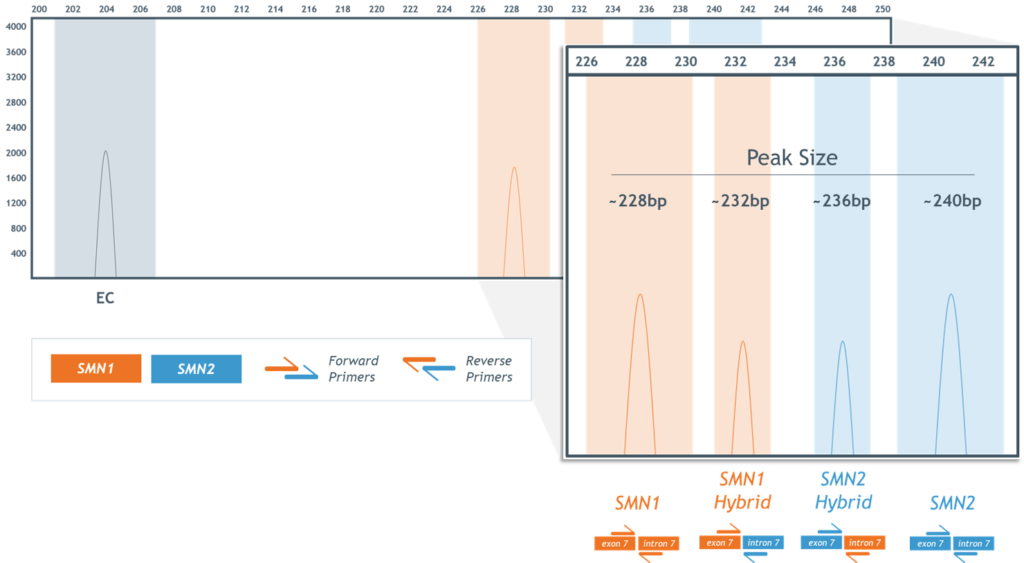
- High assay specificity permits detection of SMN1–SMN2 hybrid peaks, including those resulting from gene conversion events (Figure 4)
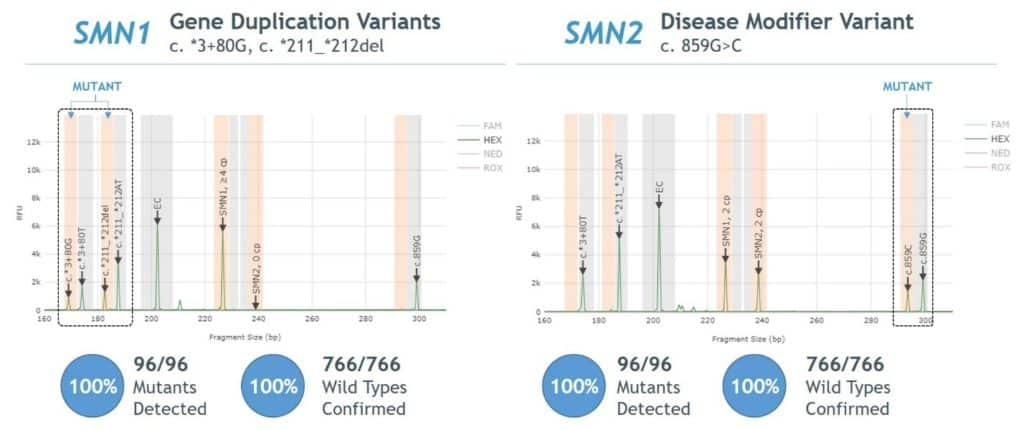
- 100% agreement between Sanger sequencing and PCR/CE SMN1/2 Plus Kit for detection of SMN1 gene duplication and SMN2 disease modifier variants (Figure 5).
Figure 1. Example Electropherogram Output – AmplideX PCR/CE SMN1/2 Plus Kit
Figure 2. AmplideX PCR/CE SMN1/2 Plus Kit Testing Workflow
Figure 3. Reproducibility of SMN1 and SMN2 copy number reporting across sites for over 500 replicates.
Figure 4. Detection and differentiation of SMN1 and SMN2 hybrid peaks.
Figure 5. Agreement between AmplideX PCR/CE SMN1/2 Plus Kit and Sanger sequencing for SMN1 gene duplication and SMN2 disease modifier variants
Additional Resources
Videos
The AmplideX PCR/CE SMN1/2 Plus Kit Illustrated
From Two Days to Four Hours: How the AmplideX PCR/CE SMN1/2 Plus Kit Provides SMN1 and SMN2 Copy Number Information and More…
Posters
A Rapid Diagnostic and Screening System for Spinal Muscular Atrophy that Reports Copy Number Changes, Single Nucleotide Variants and Small Indels
View Full Poster
Order Now
Contact Us Here To Order
Ordering Information
| Product Name | Number of Reactions | Catalog Number |
|---|---|---|
| AmplideX® PCR/CE SMN1/2 Plus Kit (RUO) | 50 | A00050 |
| AmplideX® PCR/CE SMN1/2 Plus Kit (RUO) | 100 | A00054 |
T 1-877-777-1874; 512-681-5200
F 512-681-5202
AmplideX® SMA Plus Kit

The AmplideX® SMA Plus Kit* is an in vitro nucleic acid amplification kit intended to aid in the screening of carriers for and diagnosis of spinal muscular atrophy (SMA). The kit quantifies the number of copies of exon 7 of both SMN1 and SMN2 reported as 0, 1, 2, 3, or ≥ 4 genomic copies. The kit is designed for PCR with extracted genomic DNA from human whole blood performed on standard laboratory-validated thermal cyclers, followed by resolution on a general laboratory-validated genetic analyzer or capillary electrophoresis (CE) platform. Additionally, the kit identifies chimeric genes with both SMN1 and SMN2 sequences, and detects variants SMN1 c.*3+80T>G and SMN1 c.*211_*212del, which are associated with SMN1 gene duplication and “silent carrier” status, as well as variant SMN2 c.859G>C, which is associated with a milder disease phenotype.
Order the AmplideX SMA Plus Kit
Disease Background
- General Information
- SMA is a debilitating illness resulting from the deficient production of motor neurons in the central nervous system and is a leading genetic cause of infant death.
- It is transmitted via an autosomal recessive inheritance pattern
- Diagnosing SMA
- SMA is diagnosed when no functional copies of the SMN1 gene are present
- Disease severity is inversely correlated to the number of SMN2 copies present
- Life-saving treatments must be started early, so rapid delivery of diagnostic test results is critical
- Carrier Screening
- Approximately 1 in 50 individuals is a carrier of SMA
- clinical advisory committees (American College of Medical Genetics (ACMG), American College of Obstetrics and Gynecology (ACOG)) recommend screening be available to all couples regardless of ethnicity
Features & Benefits
Reduced Complexity
Ease of data analysis and reporting
- One kit to identify SMA patients, carriers (including detection of variants associated with silent carriers), and refine disease prognosis – all from a single PCR reaction
- Similar workflow to AmplideX PCR/CE FMR1*† kit eases implementation and training
- Assay-specific software automates results reporting and streamlines data analysis
Optimized Workflow
Reduces valuable operator hands-on-time and overall turnaround time
- Diagnostic and screening results are reported in less than four hours with only 60 minutes of hands-on-time
- Scalable workflow supports high sample throughput testing
- Optimized for use on widely installed CE equipment
- Fully-kitted solution sourced from a single vendor
Quality Performance
Comprehensive analysis of SMN1 and SMN2 genes for the diagnosis and screening of SMA
- High resolution of SMN1/2 copy number across a broad range improves accuracy in identifying SMA patients and carriers
- Excellent concordance of copy number and variant results compared to multiple orthogonal test methods
*For Research Use Only. Not for use in Diagnostic procedures.
Analytical Performance
- Automated and streamlined reporting of SMN1 and SMN2 copy number and variant detection via AmplideX Reporter Software (Figure 1)
- Provide critical test results from a single reaction — and all in less than four hours (Figure 2)
- Excellent concordance of SMN1 and SMN2 copy number results to sites’ in-house methods for over 400 replicates (Figure 3)
- High assay specificity permits detection of SMN1–SMN2 hybrid peaks, including those resulting from gene conversion events (Figure 4)
- 100% agreement observed between Sanger sequencing and AmplideX SMA Plus Kit for detection of SMN1 gene duplication and SMN2 disease modifier variants (Figure 5).
Figure 1. Example Electropherogram Output for the AmplideX SMA Plus Kit
Figure 2. AmplideX SMA Plus Kit Testing Workflow
Figure 3. Excellent reproducibility of SMN1 and SMN2 copy number reporting across sites for over 400 replicates
Figure 4. Detection and identification of SMN1 and SMN2 “hybrid peaks”
Figure 5. Agreement between AmplideX SMA Plus Kit and Sanger sequencing for SMN1 gene duplication (“silent carrier”) and SMN2 disease modifier variants
Additional Resources
Videos
Analytical validation of a multiplex PCR/CE assay for simultaneous determination of SMN1/SMN2 exon 7 copy number and variant status (Milligan J, et al). Presented at ESHG 2020.2 Virtual Conference.
The AmplideX PCR/CE SMN1/2 Plus Kit Illustrated
Order Now
Contact Us Here To Order
Ordering Information
| Product Name | Number of Reactions | Catalog Number |
|---|---|---|
| AmplideX® SMA Plus Kit | 50 | A00055 |
| AmplideX® SMA Plus Kit | 100 | A00056 |
T 1-877-777-1874; 512-681-5200
F 512-681-5202