Infectious Disease Quality Controls
Our suite of custom products includes Armored RNA®, Armored RNA Quant® and Armored DNA Quant™, plasmid DNA (circular and linearized) and in vitro transcribed RNA; providing unique, target-specific controls to meet your needs.
Armored Controls have been utilized in FDA-approved assays for more than 20 years and continue to serve as important tools in the rapidly evolving space of molecular diagnostics.
What is Armored Technology?
Armored RNA® and Armored DNA™ technologies stabilize and protect nucleic acids from nuclease degradation by packaging them in a protective protein coat. Armored reagents deliver several end-user benefits, including:
Optimized Workflow
Armored RNA®, Armored RNA Quant® and Armored DNA Quant™ controls can be added directly to human matrices as an exogenous internal control for extraction with most nucleic acid isolation methods and detection in molecular assays.
Flexibility
Nuclease-resistant protein coat can also be removed by heat lysis for direct detection as a positive run control. For Armored RNA Quant® and Armored DNA Quant™, analytical quantification is performed with a validated method referencing a NIST-traceable phosphate standard.
Catalog Products
Armored RNA Quant® Respiratory Triplex Control
Seasonal respiratory pathogens can often be challenging to distinguish from one another symptomatically. Molecular detection of these different viruses offers an accurate and time- and cost-effective way to pinpoint specific targets of interest. Now, a single control can provide the sensitivity and reliability you’ve come to expect from Armored RNA.
This blend contains regions of SARS-Cov-2, Influenza A (H1N1, H3N2, and H7N9), Influenza B, Respiratory Syncytial Virus A (RSVA), Respiratory Syncytial Virus B (RSVB), and RPP30 as an internal control.
Armored RNA Quant® SARS-CoV-2 Controls
In response to COVID-19, Asuragen developed molecular diagnostic controls for multiple regions of the SARS-CoV-2 genome as well as conserved mutations in the spike protein prevalent in the UK (B.1.1.7), South African (B.1.351), and Brazilian (P.1) strains. Armored RNA Quant® controls allow for stable, reliable, and safe ways to rapidly test for the presence of Coronavirus and its variants.
Armored RNA Quant® Internal Process Control
This control is a non-specific, 1,000 base pair IVT RNA (alien/non-homologous) sequence, encapsulated in a coat of protein dimer armor which renders it resistant to degradation and therefore useful across a variety of applications. It is intended to be used as a spike-in to your sample (e.g., urine, blood, CSF, plasma/liquid biopsy) to monitor the overall efficiency of your process. This robust and versatile control allows you to measure specificity and sensitivity of your assay during development or while running samples as an on-board process control.
Armored RNA Quant® EPA-1615
In 2010, Asuragen partnered with the US EPA to create an Armored enteric control in an effort to standardize the measurement of Enterovirus and Norovirus in water by RT-qPCR. Method 1615 was published later that year and updated in 2014 as a joint publication between the US EPA National Exposure Research Laboratory and the Office of Ground Water and Drinking Water.It reduces equipment costs and time associated with labor in collection and detection of biologics from ground, reagent-grade, and surface water samples and includes the use of Armored RNA Quant® EPA-1615 as a positive reference and our Armored RNA® HGV product as an inhibition control.
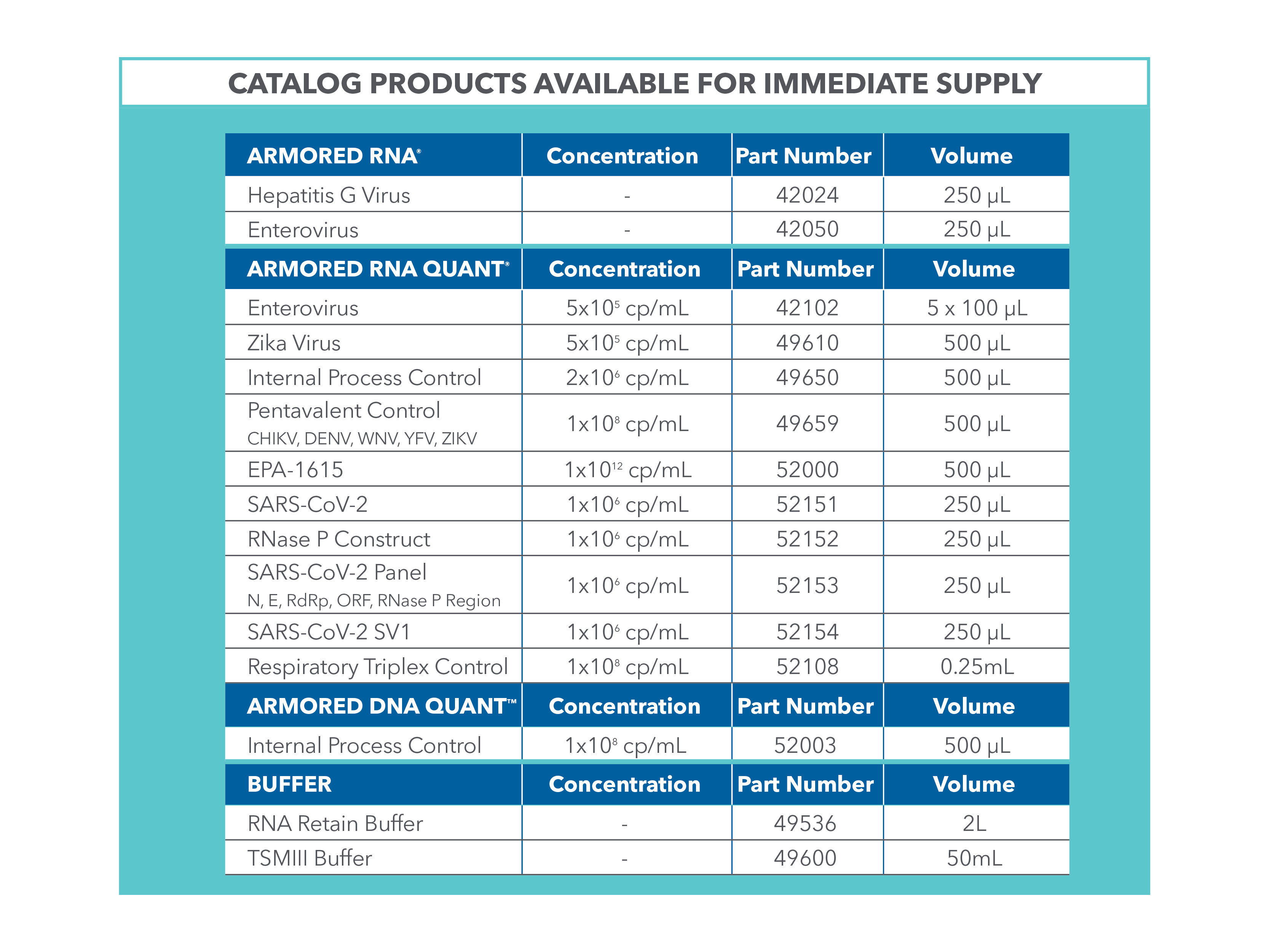
Armored Controls Catalog
*For Research Use Only. Not for use in Diagnostic procedures.
Ordering Information
To request a quote or for more information about Asuragen’s Armored catalog of molecular quality controls, please fill out the form below.
Infectious Disease Quality Controls
Our suite of custom products includes Armored RNA®, Armored RNA Quant® and Armored DNA Quant™, plasmid DNA (circular and linearized) and in vitro transcribed RNA; providing unique, target-specific controls to meet your needs.
Armored Controls have been utilized in FDA-approved assays for more than 20 years and continue to serve as important tools in the rapidly evolving space of molecular diagnostics.