AmplideX® PCR/CE CFTR Kit*

Order the AmplideX PCR/CE CFTR Kit*
Solve Cystic Fibrosis Testing Coverage Challenges
Many commercially available CFTR panels were designed using variant information from disease databases that are heavily skewed toward individuals with European ancestry, making equitable coverage for ethnically diverse populations challenging. To address these coverage gaps, Asuragen, a Bio-Techne brand’s AmplideX PCR/CE CFTR Kit was designed with diversity in mind and based on recent, large-scale population studies that include data from a range of ethnic groups more representative of the U.S. population.
Although more than 2100 CFTR variants have been documented, these variants differ in levels of pathogenicity and prevalence between ethnicities.1 ACOG guidelines state full gene sequencing is inappropriate for screening and should be reserved for specific clinical cases, so it is paramount to have a commercially available, targeted panel with a selection of variants that provides equitable coverage and is less likely to miss carriers in diverse populations.2
Figure 1.
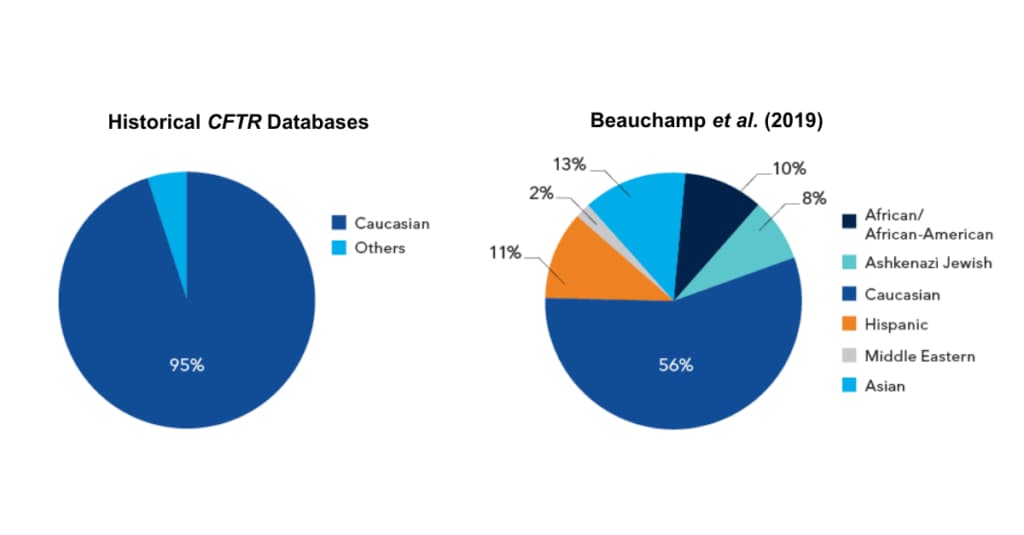
Most CFTR panels have been designed from CFTR Databases that were historically3 skewed towards one ethnicity even though the U.S. population looks more like the Beauchamp et al. database.
Asuragen Addresses Critical Coverage Gaps in CFTR Panels

Table 2.
Designed to solve complex CFTR coverage challenges to provide the broadest coverage† of the U.S. population of any available targeted kit with an easy-to-use AmplideX workflow and a complementary assay portfolio.
Features & Benefits
Reduced Complexity
- Ready-to-use test kit with quality-controlled reagents reduces pipetting steps.
- Similar workflow to AmplideX PCR/CE FMR1* and SMN1/2 Plus* kits eases implementation.
- Streamlined data analysis via AmplideX Reporter software.
Optimized Workflow
- Easy-to-use workflow designed to reduce hands-on tech time.
- Utilizes widely available laboratory PCR/CE instrumentation.
- <5 hrs from DNA to data.
Quality Results
- Built on the latest prevalence data to provide the best coverage† for all U.S. ethnicities.4
- Detects complex yet key CFTR variants (STRs, SNPs, INDELs) and resolves zygosity.
- Excellent concordance with other methods.
Analytical Performance
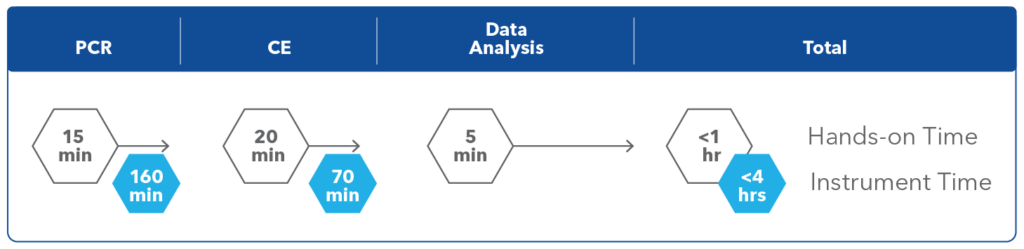
Figure 2. Hands-on time and instrument time for the AmplideX CFTR PCR/CE Kit.
Method Comparison Study
Sample level genotype agreement between AmplideX PCR/CE CFTR Kit and Reference Method.
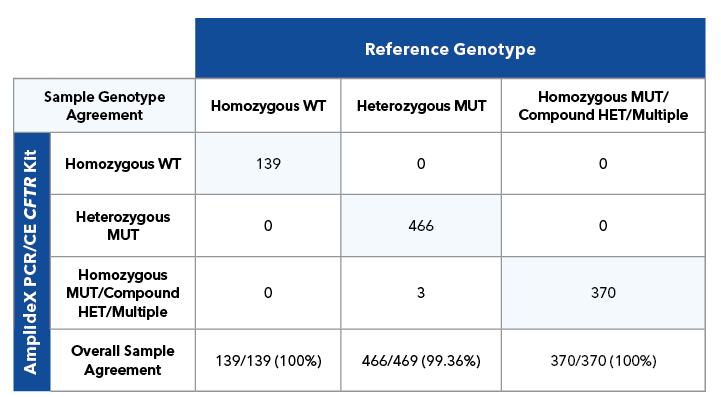
Table 3A. Sample level agreement for 146 total samples (51 DBS, 91 whole blood and 4 cell lines) run on 7 CE configurations.
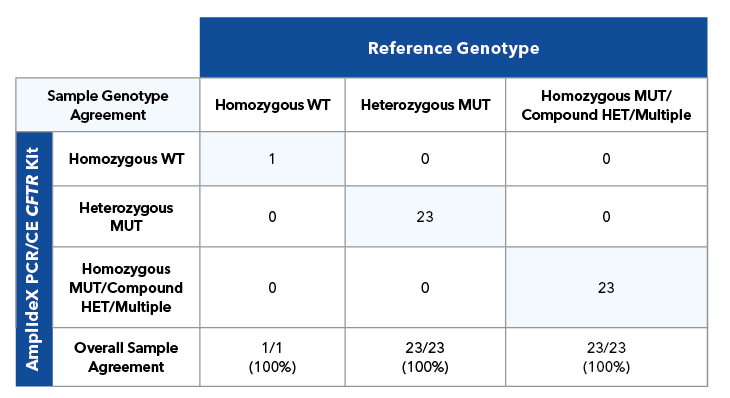
Table 3B. Sample level agreement for 47 cell line samples.
Additional Resources
- White Paper: “Making Coverage Count: Screening for CFTR Mutations in Diverse Populations with Effective Variant Panels”
- Webinar: Do More with Less: Better Genetic Answers in a Streamlined Analysis Workflow
- Video: Answers for All: Addressing Coverage Gaps in Current CFTR testing
- Video: The AmplideX PCR/CE CFTR Kit Illustrated
- Corporate Workshop: Practical and Reliable CFTR Variant Detection for Diverse Populations with the AmplideX® PCR-CE CFTR Kit
Order Now
Contact Us Here To Order
Ordering
| Product Name | Number of Reactions | Catalog Number |
|---|---|---|
| AmplideX® PCR/CE CFTR Kit** | 50 | A00519 |
| AmplideX® PCR/CE CFTR Kit** | 100 | A00520 |
References
- Russo ML. UpToDate. 2020.
- Opinion 691. ACOG. 2017
- Sosnay et al Nat Genet. 2013 Oct; 45(10): 1160–1167
- Based on data from Beauchamp KA, et al. Genet Med. 2019.
*For Research Use Only. Not for use in Diagnostic procedures.
AmplideX® PCR/CE CFTR Kit*
Order the AmplideX PCR/CE CFTR Kit*
Solve Cystic Fibrosis Testing Coverage Challenges
Many commercially available CFTR panels were designed using variant information from disease databases that are heavily skewed toward individuals with European ancestry, making equitable coverage for ethnically diverse populations challenging. To address these coverage gaps, Asuragen, a Bio-Techne brand’s AmplideX PCR/CE CFTR Kit was designed with diversity in mind and based on recent, large-scale population studies that include data from a range of ethnic groups more representative of the U.S. population.
Although more than 2100 CFTR variants have been documented, these variants differ in levels of pathogenicity and prevalence between ethnicities.1 ACOG guidelines state full gene sequencing is inappropriate for screening and should be reserved for specific clinical cases, so it is paramount to have a commercially available, targeted panel with a selection of variants that provides equitable coverage and is less likely to miss carriers in diverse populations.2
Figure 1.

Most CFTR panels have been designed from CFTR Databases that were historically3 skewed towards one ethnicity even though the U.S. population looks more like the Beauchamp et al. database.
Asuragen Addresses Critical Coverage Gaps in CFTR Panels

Table 2.
Designed to solve complex CFTR coverage challenges to provide the broadest coverage† of the U.S. population of any available targeted kit with an easy-to-use AmplideX workflow and a complementary assay portfolio.

Solve Cystic Fibrosis Testing Coverage Challenges
Many commercially available CFTR panels were designed using variant information from disease databases that are heavily skewed toward individuals with European ancestry, making equitable coverage for ethnically diverse populations challenging. To address these coverage gaps, Asuragen, a Bio-Techne brand’s AmplideX PCR/CE CFTR Kit was designed with diversity in mind and based on recent, large-scale population studies that include data from a range of ethnic groups more representative of the U.S. population.
Although more than 2100 CFTR variants have been documented, these variants differ in levels of pathogenicity and prevalence between ethnicities.1 ACOG guidelines state full gene sequencing is inappropriate for screening and should be reserved for specific clinical cases, so it is paramount to have a commercially available, targeted panel with a selection of variants that provides equitable coverage and is less likely to miss carriers in diverse populations.2
Figure 1.

Most CFTR panels have been designed from this type of CFTR2 Database even though the U.S. population looks more like the Beauhchamp et al. database.
Asuragen Addresses Critical Coverage Gaps in CFTR Panels

Table 2.
Designed to solve complex CFTR coverage challenges to provide the broadest coverage† of the U.S. population of any available targeted kit with an easy-to-use AmplideX workflow and a complementary assay portfolio.

Features & Benefits
Reduced Complexity
- Ready-to-use test kit with quality-controlled reagents reduces pipetting steps.
- Similar workflow to AmplideX PCR/CE FMR1* and SMN1/2 Plus* kits eases implementation.
- Streamlined data analysis via AmplideX Reporter software.
Optimized Workflow
- Easy-to-use workflow designed to reduce hands-on tech time.
- Utilizes widely available laboratory PCR/CE instrumentation.
- <5 hrs from DNA to data.
Quality Results
- Built on the latest prevalence data to provide the best coverage† for all U.S. ethnicities.4
- Detects complex yet key CFTR variants (STRs, SNPs, INDELs) and resolves zygosity.
- Excellent concordance with other methods.
Analytical Performance
Figure 2. Hands-on time and instrument time for the AmplideX CFTR PCR/CE Kit.

Method Comparison Study
Sample level genotype agreement between AmplideX PCR/CE CFTR Kit and Reference Method.

Table 3A. Sample level agreement for 146 total samples (51 DBS, 91 whole blood and 4 cell lines) run on 7 CE configurations.

Table 3B. Sample level agreement for 47 cell line samples.
Additional Resources
- White Paper: “Making Coverage Count: Screening for CFTR Mutations in Diverse Populations with Effective Variant Panels”
- Webinar: Do More with Less: Better Genetic Answers in a Streamlined Analysis Workflow
- Video: Answers for All: Addressing Coverage Gaps in Current CFTR testing
- Video: The AmplideX PCR/CE CFTR Kit Illustrated
- Corporate Workshop: Practical and Reliable CFTR Variant Detection for Diverse Populations with the AmplideX® PCR-CE CFTR Kit
Order Now
Ordering
| Product Name | Number of Reactions | Catalog Number |
|---|---|---|
| AmplideX® PCR/CE CFTR Kit** | 50 | A00519 |
| AmplideX® PCR/CE CFTR Kit** | 100 | A00520 |
References
- Russo ML. UpToDate. 2020.
- Opinion 691. ACOG. 2017
- Sosnay et al Nat Genet. 2013 Oct; 45(10): 1160–1167
- Based on data from Beauchamp KA, et al. Genet Med. 2019.
*For Research Use Only. Not for use in Diagnostic procedures.
