QuantideX® qPCR BCR-ABL IS Kit
A Scalable Workflow for BCR-ABL Monitoring in CML Patients
Confidently deliver results with the first FDA-cleared chronic myeloid leukemia (CML) monitoring assay for BCR-ABL Major breakpoints (e13a2, e14a2). Your lab is empowered to provide reliable results easily with direct reporting on the International Scale and deep clinical sensitivity of 0.002% IS (MR4.7). Scalable, high-throughput testing is enabled by QuantideX multiplex assay design and included analysis software.
Order the QuantideX qPCR BCR-ABL IS Kit
Features & Benefits
The QuantideX qPCR BCR-ABL IS Kit’s unprecedented level of sensitivity coupled to a simple-to-run, singlicate test, allows labs to reliably and reproducibly monitor much deeper molecular response.
Reduced Complexity
Ease-of-data analysis and reporting for your laboratory:
- Direct reporting on the IS with four calibrators traceable to the WHO primary standards
- All-in-one kit and software from a single vendor
Optimized Workflow
Valuable operator hands-on time has been significantly reduced through:
- Multiplex design detects BCR-ABL and ABL in the same reaction, with ability to run up to 49 samples per plate
- Simplified reporting with included QuantideX Reporter software
Quality Results
Detecting BCR-ABL Transcripts robustly, reliably with a highly sensitive assay:
- Limit of Detection of MR4.7 (0.002% IS) was confirmed in clinical human blood samples, not cell lines
- Rigorous validation with FDA-clearance, single- and multi-site reproducibility studies, and seven years in clinical market use
Analytical Characteristics
Figure 1. Highly sensitive results

LOD of 95% detection at MR4.7 was determined from 1,678 valid data points from human RNA, from 40 test runs by four operators over two kit lots, 15 days, and four instruments.†
Table 1. Highly reproducible results
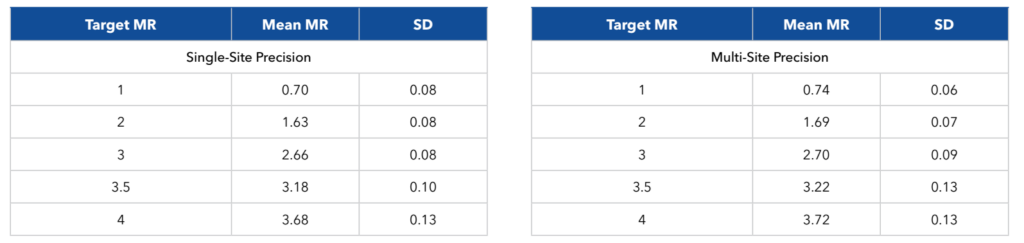
Single- and multi-site reproducibility studies† demonstrated minimal variation across the assay dynamic range, with a maximum standard deviation of 0.13 MR units.
Scalable Results
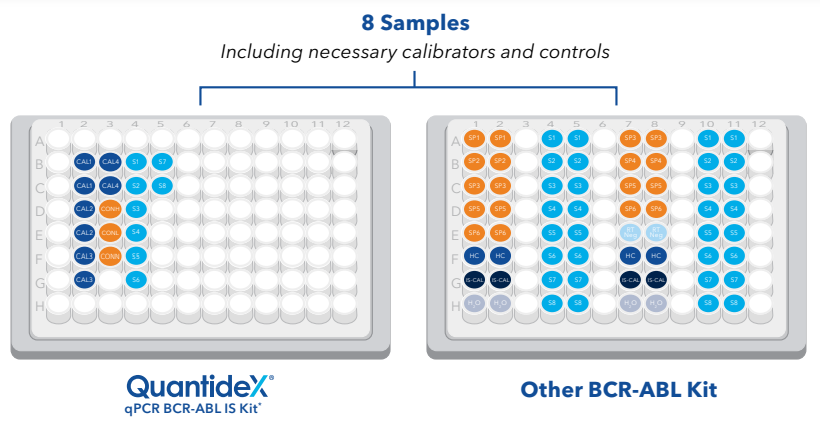
Only 19 reactions are required for eight samples tested with the QuantideX assay, compared to 64 reactions for an assay with
a simplex design and samples run in duplicate.
The QuantideX multiplex workflow allows up to 49 samples in singleton per plate, enabling efficient, high-throughput testing.
Additional Resources
Videos
View our video resources.
Posters
- Method Comparison Between Two Commercially Available BCR-ABL1 Quantitative Kits. Lara A, Beldorth I, Jefferson K, Brown J. AMP 2021.
- Validation of BCR-ABL1 Test Performance from Whole Blood Stored up to 72 Hours Facilitates Operational Flexibility and Expanding Locally Managed CML Monitoring. Beldorth I, Masson K, Fahey M, Ruskin A, Andruss B, Brown J. John Goldman ESH-iCMLf 2018 (European School of Hematology International CML Foundation).
- BCR-ABL1 Molecular Responses at 12-18 Months Predict Long-Term Event-Free Survival in Patients with Tyrosine Kinase Inhibitor (TKI)-Treated Chronic Myelogenous Leukemia (CML). Press R, Watt C, Cai L, Van Deerlin V, Roth J, Gentile C, Loren A, and Eickelberg G. AMP 2016.
Publications and Articles
- Analytical Validation of a Highly Sensitive, Multiplexed Chronic Myeloid Leukemia Monitoring System Targeting BCR-ABL1 RNA. Brown et al. J Mol Diagn. 2019 Jul;21(4):718-733.
- As CML Outcomes Improve, Demand Rises for BCR::ABL1 Testing. Brown, J. Clinical Lab Products. 2023.
- Asuragen’s QuantideX® qPCR BCR-ABL IS Kit Receives FDA Premarket Clearance for Monitoring Minimal Residual Disease in Chronic Myeloid Leukemia. Business Wire. 2016.
The test is not intended for the diagnosis of CML or for monitoring rare transcripts resulting from t(9;22).
Order Now
Contact Us Here To Order
Ordering
| Product Name | Number of Reactions | Catalog Number |
|---|---|---|
| QuantideX® qPCR BCR-ABL IS Kit | 60 | 49574 |
*US-IVD †Brown JT, et al. J Mol Diagn. 2019 Jul;21(4):718-733.
QuantideX® qPCR BCR-ABL IS Kit

Confidently deliver results with the first C-certified IVDR chronic myeloid leukaemia (CML) monitoring assay with BCR ABL Major breakpoints (e13a2, e14a2). With direct reporting on the International Scale (IS) and deep clinical sensitivity of 0.002% (MR 4.7), laboratories can assess the deepest molecular response with unprecedented ease and deliver the results physicians and patients rely on. The QuantideX multiplex assay design and included analysis software render reporting CML monitoring results both accurate and quick, with results in ~4 hours.
Features & Benefits
The Quantidex qPCR BCR-ABL IS Kit provides labs with a robust and reliable Class C IVDR-certified method for monitoring patients with CML.
Reduced Complexity
- Direct reporting on the IS with four calibrators traceable to the WHO primary standards
- Ready-to-use kit with included RT reagents and calibrators, controls and software module
Optimized Workflow
- Multiplex design detects BCR-ABL and ABL in the same reaction, with the ability to run up to 49 clinical samples per plate
- Simplified reporting with included QuantideX® Reporter Software
Quality Results
- Limit of Detection of MR 4.7 (0.002% IS) confirmed in clinical human blood samples, not cell lines
- Trusted, reproducible results with Class C IVDR certification
Analytical Characteristics
Figure 1. Highly sensitive results

LOD of 95% detection at MR4.7 was determined from 1,678 valid data points from human RNA, from 40 test runs by four operators over two kit lots, 15 days, and four instruments.†
Minimal Variability Across Entire Dynamic Range of %IS Values

Figure 2. Precision was evaluated by using 5 different levels of positive specimen, tested by 3 operators over 20 runs. Each level was tested 40 times to obtain Standard Deviations
Comparison of a plate layout for 8 sample run
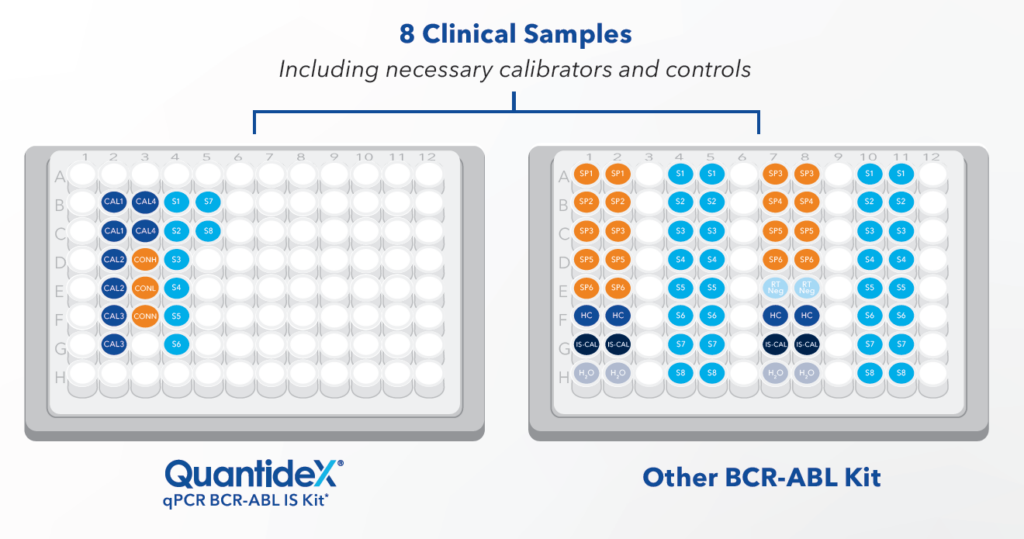
Figure 3. Only 19 reactions are required for eight samples tested with the QuantideX assay, compared to 64 reactions for an assay with
a simplex design and samples run in duplicate. The QuantideX multiplex workflow allows up to 49 samples in singleton per plate, enabling efficient, high-throughput testing.
Additional Resources
Videos
View our video resources.
Posters
- QuantideX qPCR BCR-ABL IS Kit and ipsogen BCR-ABL1 Mbcr IS-MMR Kit Yield Highly Correlated Results. Lara A, Beldorth I, Jefferson K, Brown J. John Goldman ESH-iCMLf 2021 (European School of Hematology International CML Foundation).
- Validation of BCR-ABL1 Test Performance from Whole Blood Stored up to 72 Hours Facilitates Operational Flexibility and Expanding Locally Managed CML Monitoring. Press R, Watt C, Cai L, Van Deerlin V, Roth J, Gentile C, Loren A, and Eickelberg G. AMP 2016.
- BCR-ABL1 Molecular Responses at 12-18 Months Predict Long-Term Event-Free Survival in Patients with Tyrosine Kinase Inhibitor (TKI)-Treated Chronic Myelogenous Leukemia (CML). Beldorth I, Masson K, Fahey M, Ruskin A, Andruss B, Brown J. John Goldman ESH-iCMLf 2018 (European School of Hematology International CML Foundation).
Publications and Articles
- Analytical Validation of a Highly Sensitive, Multiplexed Chronic Myeloid Leukemia Monitoring System Targeting BCR-ABL1 RNA. Brown et al. J Mol Diagn. 2019 Jul;21(4):718-733.
- As CML Outcomes Improve, Demand Rises for BCR::ABL1 Testing. Brown, J. Clinical Lab Products. 2023.
- Asuragen, Inc. Announces Launch Of Highly Sensitive CE-IVD BCR-ABL Assay At The European Hematology Association Annual Congress. BioSpace. 2015.
Browse all Publications
The test is not intended for the diagnosis of CML or for monitoring rare transcripts resulting from t(9;22).
Order Now
Ordering
| Product Name | Number of Reactions | Catalog Number |
|---|---|---|
| QuantideX® qPCR BCR-ABL IS Kit | 60 | 86003 |
*US-IVD †Brown JT, et al. J Mol Diagn. 2019 Jul;21(4):718-733.

