QuantideX® qPCR ESR1 exoMutation Kit*

Bringing Ultra High-Sensitivity to ESR1 Mutation Detection
The QuantideX qPCR ESR1 exoMutation Kit is the first multianalyte liquid biopsy assay tailored for detecting ESR1 mutations. Designed to provide highly sensitive detection of the 11 most prevalent1 ESR1 variants in plasma. The kit utilizes both cell-free DNA (cfDNA) and exosomal RNA (exoRNA), which are isolated using the ExoLution Plus cfDNA + exoRNA Isolation Kit*, provided with the ESR1 exoMutation Kit. The kit offers a novel approach to achieving more meaningful breast cancer insights.
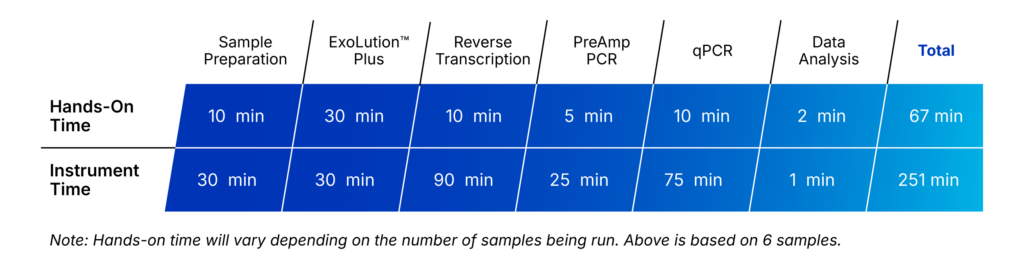
Complete QuantideX qPCR ESR1 Workflow in a Single Day
The QuantideX qPCR ESR1 exoMutation Kit is paired with the ExoLution™ Plus cfDNA + exoRNA Isolation Kit, leveraging proprietary chemistries to co-isolate cfDNA + exoRNA in a single workflow. ExoLution Plus includes all necessary reagents and consumables for high-quality multianalyte isolation to power downstream mutation testing.
Figure 1: Combined Workflow for the QuantideX qPCR ESR1 exoMutation Kit and ExoLution Plus cfDNA and exoRNA Isolation Kit
ESR1 Mutations are the Leading Cause of Primary Treatment Resistance in HR+ mBC
The ESR1 gene encodes for estrogen receptor alpha (ERα), and mutations in its ligand-binding domain result in constitutive activation of the estrogen receptor, causing overactive downstream signaling. ESR1 mutations have emerged as a key biomarker in determining resistance to therapy in hormone receptor-positive (HR+), metastatic breast cancer (mBC) patients. While these mutations are exceptionally rare in treatment-naïve patients, they are prevalent in up to 40% of mBC patients with prolonged exposure to endocrine therapy (ET) and lead to treatment failure and ultimately, disease progression2. Recent and ongoing clinical trials aim to demonstrate that changing therapy at the first appearance of ESR1 mutations results in marked improvements in reduction of disease progression. Emerging novel therapies targeting ESR1-mutant disease offer a promising solution to overcoming this mechanism of primary therapy resistance.
Optimizing Data Reporting with Automated Software Feature
The QuantideX qPCR ESR1 exoMutation Kit’s ready-to-use design doesn’t stop at the bench. Our push-button, automated software, created to run on commonly used operating systems, takes the guesswork and hands-on time out of data analysis. The software module is included with the assay and does not require separate purchases or licenses.
Probit analysis of synthetic DNA (0, 1, 3, 5, 10 copies/reaction, 20 replicates of each) titrated in a background of presumed normal DNA (10,000 total copies).
| Target | LOD (%VAF) |
|---|---|
| D538G | 0.082% |
| S463P | 0.066% |
| Y537S | 0.025% |
| Y537C | 0.029% |
| Y537N | 0.025% |
| Y537D | 0.029% |
| E380Q | 0.028% |
| L536R | 0.028% |
| L536H | 0.041% |
| L536P | 0.028% |
| V422del | 0.030% |
Leveraging Exosomes for a Differentiated Approach
Whereas cfDNA is released exclusively from dying cells and is in relatively low abundance, all living cells actively secrete tens of thousands of exosomes every day releasing a great abundance of biological material into bodily fluids. Exosomes contain exosomal RNA, which provides a dynamic snapshot of the body’s transcriptome. By co-analyzing exoRNA with cfDNA via liquid biopsy, we can unlock highly sensitive, minimally invasive biomarker detection using established qPCR testing technologies. Utilizing this approach, Asuragen has developed the first multianalyte qPCR-based liquid biopsy assay for the detection of ESR1 mutations from plasma, empowering molecular laboratories to participate in this new frontier of clinical research and push the boundaries of conventional qPCR. The QuantideX qPCR ESR1 exoMutation Kit is designed to provide highly sensitive detection of 11 of the most prevalent ESR1 variants in plasma, offering a novel approach to achieving more meaningful breast cancer insights.
High Analytical Specificity Observed Across Both K2EDTA and PAXgene Tubes
Evaluation of target analytical specificity (exclusivity) was determined on plasma procured from presumed normal samples.
- Utilized ExoLution Plus cfDNA + exoRNA Isolation Kit workflow for nucleic acid isolation
- Support of multiple collection tubes provides more flexibility across laboratory operations
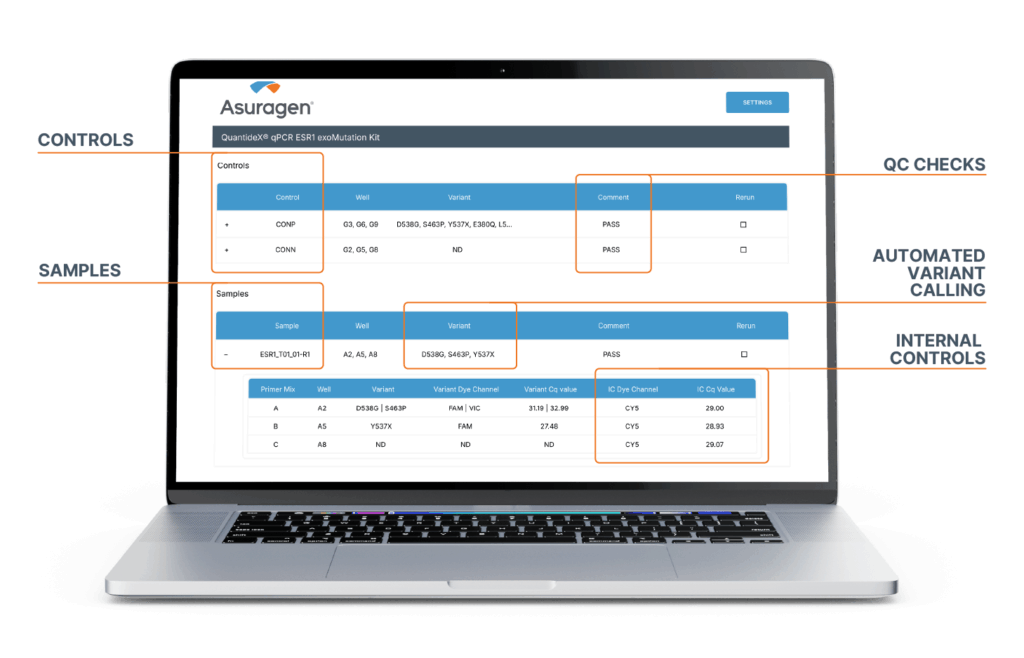
Software Enables Comprehensive, Push-Button Analysis and QC
QuantideX qPCR ESR1 exoMutation Analysis Module*
- Automated variant calling
- Batch Control QCs
- Click on the “+” on the left side of table to drill down to Ct (Cq) values
- Export results in LIMS-compatible format (CSV)
Product Description
| Category | Description |
|---|---|
| Coverage | 11 ligand-binding domain mutations: E380Q; V422del; S463P; L536H/P/R (X); Y537S/N/C/D (X); D538G |
| Capacity | 50 Reactions |
| Analyte | cfDNA + exoRNA |
| Sample | 2 mL Plasma |
| NA Isolation | ExoLution™ Plus |
| Assay Sensitivity | 0.03-0.08% VAF (~5 copies/mL) |
| Workflow | Sample-to-answer in <8 hours |
| Within Sample Control (IC1, IC2 & IC3) | The IC informs the user if they have added adequate sample into each of the testing wells |
| Batch Controls | The Positive Control (CONP) is a DNA based control for 6 of the 11 targeted mutations. Each mix contains 2 mutations as a positive control.
|
| Batch Controls | The Negative Control (CONN) is an RNA based negative control. It acts as:
|
Resources
Watch the Workshop: ESR1 & Beyond: Leveraging Exosomes for Highly-Sensitive Variant Detection on qPCR | Presented at the Association for Molecular Pathology Annual Meeting 2024
SABCS Poster: Verification of a Highly Sensitive, Circulating Cell-Free DNA and Exosomal RNA RT-qPCR Assay System to Monitor ESR1 Mutations from a Liquid Biopsy | Kevin Kelnar, Blaine Caughron, Holli Dale….SABCS 2024
View the Poster: Verification of an RT-qPCR Assay System for Liquid Biopsy Surveillance of Treatment-Resistant ESR1 Mutations | Presented at the Association for Molecular Pathology Annual Meeting & Expo 2024
Learn more about the Power of Exosomes
Learn more about the Exosome Platforms capabilities for Cancer Mutation Detection
An Exosome-based ESR1 Monitoring RT-qPCR Kit That Rapidly and Accurately Detects Acquired Resistance Variants at ≤ 0.1% Frequency in Liquid Biopsy Samples | Sarah Statt, Julie R Thibert, Melissa Church, Jamie Myers1, Holli Dale, Blaine Caughron, Kevin Kelnar, and Brian Haynes
Ordering
| Product Name | Number of Reactions | Catalog Number |
|---|---|---|
| QuantideX® qPCR ESR1 exoMutation Kit | 50 | A01052 |
| Quantidex ExoLution Plus Kit | 50 | A00669 |
Contact Us
Contact us to learn more about the QuantideX qPCR ESR1 ExoMutation Kit
Disclaimer
*For Research Use Only. Not for use in diagnostic procedures.
**ABI QuantStudio 5 Dx
- Breast Cancer Res. 2021 Aug 15;23(1):85. 2. Lancet Oncol. 2022 Nov;23(11):1367-1377.
- Hartkopf, AD et al. Breast Care 2020;15:347-354: DOI:10.1159/000508675
QuantideX® qPCR ESR1 exoMutation Kit*

Bringing Ultra High-Sensitivity to ESR1 Mutation Detection
The QuantideX qPCR ESR1 exoMutation Kit is the first multianalyte liquid biopsy assay tailored for detecting ESR1 mutations. Designed to provide highly sensitive detection of the 11 most prevalent1 ESR1 variants in plasma. The kit utilizes both cell-free DNA (cfDNA) and exosomal RNA (exoRNA), which are isolated using the ExoLution Plus cfDNA + exoRNA Isolation Kit*, provided with the ESR1 exoMutation Kit. The kit offers a novel approach to achieving more meaningful breast cancer insights.
Complete QuantideX qPCR ESR1 Workflow in a Single Day
The QuantideX qPCR ESR1 exoMutation Kit is paired with the ExoLution™ Plus cfDNA + exoRNA Isolation Kit, leveraging proprietary chemistries to co-isolate cfDNA + exoRNA in a single workflow. ExoLution Plus includes all necessary reagents and consumables for high-quality multianalyte isolation to power downstream mutation testing.
Figure 1: Combined Workflow for the QuantideX qPCR ESR1 exoMutation Kit and ExoLution Plus cfDNA and exoRNA Isolation Kit
ESR1 Mutations are the Leading Cause of Primary Treatment Resistance in HR+ mBC
The ESR1 gene encodes for estrogen receptor alpha (ERα), and mutations in its ligand-binding domain result in constitutive activation of the estrogen receptor, causing overactive downstream signaling. ESR1 mutations have emerged as a key biomarker in determining resistance to therapy in hormone receptor-positive (HR+), metastatic breast cancer (mBC) patients. While these mutations are exceptionally rare in treatment-naïve patients, they are prevalent in up to 40% of mBC patients with prolonged exposure to endocrine therapy (ET) and lead to treatment failure and ultimately, disease progression2. Recent and ongoing clinical trials aim to demonstrate that changing therapy at the first appearance of ESR1 mutations results in marked improvements in reduction of disease progression. Emerging novel therapies targeting ESR1-mutant disease offer a promising solution to overcoming this mechanism of primary therapy resistance.
Optimizing Data Reporting with Automated Software Feature
The QuantideX qPCR ESR1 exoMutation Kit’s ready-to-use design doesn’t stop at the bench. Our push-button, automated software, created to run on commonly used operating systems, takes the guesswork and hands-on time out of data analysis. The software module is included with the assay and does not require separate purchases or licenses.
Probit analysis of synthetic DNA (0, 1, 3, 5, 10 copies/reaction, 20 replicates of each) titrated in a background of presumed normal DNA (10,000 total copies).
| Target | LOD (%VAF) |
|---|---|
| D538G | 0.082% |
| S463P | 0.066% |
| Y537S | 0.025% |
| Y537C | 0.029% |
| Y537N | 0.025% |
| Y537D | 0.029% |
| E380Q | 0.028% |
| L536R | 0.028% |
| L536H | 0.041% |
| L536P | 0.028% |
| V422del | 0.030% |
Leveraging Exosomes for a Differentiated Approach
Whereas cfDNA is released exclusively from dying cells and is in relatively low abundance, all living cells actively secrete tens of thousands of exosomes every day releasing a great abundance of biological material into bodily fluids. Exosomes contain exosomal RNA, which provides a dynamic snapshot of the body’s transcriptome. By co-analyzing exoRNA with cfDNA via liquid biopsy, we can unlock highly sensitive, minimally invasive biomarker detection using established qPCR testing technologies. Utilizing this approach, Asuragen has developed the first multianalyte qPCR-based liquid biopsy assay for the detection of ESR1 mutations from plasma, empowering molecular laboratories to participate in this new frontier of clinical research and push the boundaries of conventional qPCR. The QuantideX qPCR ESR1 exoMutation Kit is designed to provide highly sensitive detection of 11 of the most prevalent ESR1 variants in plasma, offering a novel approach to achieving more meaningful breast cancer insights.
High Analytical Specificity Observed Across Both K2EDTA and PAXgene Tubes
Evaluation of target analytical specificity (exclusivity) was determined on plasma procured from presumed normal samples.
- Utilized ExoLution Plus cfDNA + exoRNA Isolation Kit workflow for nucleic acid isolation
- Support of multiple collection tubes provides more flexibility across laboratory operations
Software Enables Comprehensive, Push-Button Analysis and QC
QuantideX qPCR ESR1 exoMutation Analysis Module*
- Automated variant calling
- Batch Control QCs
- Click on the “+” on the left side of table to drill down to Ct (Cq) values
- Export results in LIMS-compatible format (CSV)
Product Description
| Category | Description |
|---|---|
| Coverage | 11 ligand-binding domain mutations: E380Q; V422del; S463P; L536H/P/R (X); Y537S/N/C/D (X); D538G |
| Capacity | 50 Reactions |
| Analyte | cfDNA + exoRNA |
| Sample | 2 mL Plasma |
| NA Isolation | ExoLution™ Plus |
| Assay Sensitivity | 0.03-0.08% VAF (~5 copies/mL) |
| Workflow | Sample-to-answer in <8 hours |
| Within Sample Control (IC1, IC2 & IC3) | The IC informs the user if they have added adequate sample into each of the testing wells |
| Batch Controls | The Positive Control (CONP) is a DNA based control for 6 of the 11 targeted mutations. Each mix contains 2 mutations as a positive control.
|
| Batch Controls | The Negative Control (CONN) is an RNA based negative control. It acts as:
|
Resources
Watch the Workshop: ESR1 & Beyond: Leveraging Exosomes for Highly-Sensitive Variant Detection on qPCR | Presented at the Association for Molecular Pathology Annual Meeting 2024
SABCS Poster: Verification of a Highly Sensitive, Circulating Cell-Free DNA and Exosomal RNA RT-qPCR Assay System to Monitor ESR1 Mutations from a Liquid Biopsy | Kevin Kelnar, Blaine Caughron, Holli Dale….SABCS 2024
View the Poster: Verification of an RT-qPCR Assay System for Liquid Biopsy Surveillance of Treatment-Resistant ESR1 Mutations | Presented at the Association for Molecular Pathology Annual Meeting & Expo 2024
Learn more about the Power of Exosomes
Learn more about the Exosome Platforms capabilities for Cancer Mutation Detection
An Exosome-based ESR1 Monitoring RT-qPCR Kit That Rapidly and Accurately Detects Acquired Resistance Variants at ≤ 0.1% Frequency in Liquid Biopsy Samples | Sarah Statt, Julie R Thibert, Melissa Church, Jamie Myers1, Holli Dale, Blaine Caughron, Kevin Kelnar, and Brian Haynes
Ordering
| Product Name | Number of Reactions | Catalog Number |
|---|---|---|
| QuantideX® qPCR ESR1 exoMutation Kit | 50 | A01052 |
| Quantidex ExoLution Plus Kit | 50 | A00669 |
Contact Us
Contact us to learn more about the QuantideX qPCR ESR1 ExoMutation Kit