CML Patients Deserve Better Answers
Welcome
A cancer diagnosis is scary, but today, with treatment and careful monitoring, most patients living with chronic myeloid leukemia (CML) have near-normal life expectancies. CML is manageable thanks to advances in testing and treatment options.3
This site is designed to give CML patients and their loved ones the resources to feel empowered and get the best answers they can. The terminology is based on guidelines in the United States. Variations exist in other regions, and guidelines change over time, so it’s critical to consult with a doctor for any questions or concerns you may have.
CML Overview
What is Chronic Myeloid Leukemia (CML)?
Chronic Myeloid Leukemia (CML) is a type of cancer that starts in the bone marrow. It results in immature white blood cells growing out of control. This guide covers the chronic phase of CML, which is when most patients are diagnosed.
What causes CML?
CML comes from a random mutation in genes. It’s not likely that you did anything that led to this disease. It is more common as people age, and it’s slightly more common in men than in women. In rare instances, it can come from being exposed to high doses of radiation. Otherwise, no other risk factors are known.
CML is not passed down through family genes. It also can’t be passed around like a cold, so it wasn’t caught from anyone else.
It’s simply a random event that causes CML.
How common is CML?
In the US, there are more than 8,000 new cases of CML diagnosed every year. It accounts for about 15% of all types of leukemia (blood cancers) in adults. It can happen at any age, but the average age at diagnosis is about 64.1
The number of people living with CML has increased over the years. This is partly good news because it means people with CML are living longer due to advances in treatment and monitoring. In the US, it is estimated that there will be at least 140,000 people living with CML by 2030.2
How is CML diagnosed?
CML is usually suspected based on routine blood work, which is one reason why it’s important to have an annual checkup. A few people might experience some symptoms, like fatigue or night sweats, but most don’t.
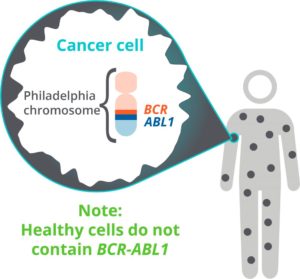
If CML is suspected on routine bloodwork, it will need to be confirmed with multiple tests requiring blood and bone marrow samples. An important part of the diagnosis is identifying the presence of the “Philadelphia chromosome”, which everyone with CML has. The Philadelphia chromosome is formed when your DNA randomly breaks, and your body puts it back together improperly. Your clinician will also need to determine the point at which the DNA broke, sometimes called a “breakpoint”. This guide is written about the specific breakpoint that about 99% of CML patients have—hence, it’s called the “Major” breakpoint.
Once CML is confirmed, it’s extremely important to get treatment and monitoring—even if you don’t feel sick. The excess growth of cancer cells eventually crowds out healthy cells, which can lead to severe sickness and death.3
How is CML treated?
Targeted medications are available. CML is typically treated with a class of medication called tyrosine kinase inhibitors (TKIs).3 The first TKI on the market is called Gleevec® (Novartis), or by its generic name imatinib. It was featured on the cover of Time magazine in 2001 because it was such a revolutionary breakthrough for CML treatment.4 Since then, additional TKIs have been approved. You will need to work with your doctor to determine the best medication and dosage specifically for you.
CML Monitoring
Why do CML patients need monitoring?
Monitoring is critical since it’s a key part of how most CML cases were transformed from life-threatening to manageable. The purpose of monitoring is to determine how well the medication is doing its job. Monitoring provides you with reassurance that the disease is responding to treatment, and a good response may predict a good outcome over the years.
As a CML patient, if you do not monitor your disease, you run the risk of failing to see that the disease might not be responding to treatment. Or you might fail to see that the disease had been responding to treatment and then quit responding—which could be a sign of relapse. This can be due to different underlying causes, such as developing resistance. Thankfully, if your medication isn’t working, your physician may be able to switch you to a more effective treatment. For all of these important reasons, monitoring is essential to tell how your current treatment is working. The good news is that most monitoring today only requires a visit to the doctor and a blood sample.3
In addition, studies have shown that monitoring early and on the recommended schedule reduces overall healthcare costs.5,12
How is CML monitoring done?
 Monitoring uses multiple lab tests.3 Initially, general blood work could give a rough idea of response to treatment. Since CML was likely suspected based on a high white blood cell count, seeing it return to a normal level is a good start—but it’s not enough (see “CHR”). Next, tests might be run to look for the Philadelphia chromosome itself which should disappear over the course of treatment (see “CCyR”). Though this is an even better sign, it’s still not sufficient to be sure that the leukemia is at low enough levels in your body.
Monitoring uses multiple lab tests.3 Initially, general blood work could give a rough idea of response to treatment. Since CML was likely suspected based on a high white blood cell count, seeing it return to a normal level is a good start—but it’s not enough (see “CHR”). Next, tests might be run to look for the Philadelphia chromosome itself which should disappear over the course of treatment (see “CCyR”). Though this is an even better sign, it’s still not sufficient to be sure that the leukemia is at low enough levels in your body.
In order to measure very small amounts of leukemia molecules that are in the body, molecular methods (e.g. PCR, qPCR, and RT-qPCR) are used by many laboratories. PCR can be a very sensitive and accurate laboratory method used to estimate the amount of leukemia molecules, called “BCR-ABL1”, in the body. These molecules come from the Philadelphia chromosome. So, the lower the amount of BCR-ABL1, the less leukemia is present. The good news is that the PCR test is performed with a blood sample.
PCR was initially used for CML because of its potential to detect disease at very low levels. Once the blood cell counts (CHR) and chromosome counts (CCyR) were back to normal, monitoring by PCR would begin. However, now it’s important to be sure that PCR is ordered from the very start of your diagnosis and throughout lifelong monitoring. This is because your doctor needs to be certain that you have the common form of BCR-ABL1 that can be monitored. Rare forms exist that cannot be monitored by typical PCR tests.3
Finally, be aware that not all PCR tests are created equal. Some may give more consistent, accurate results than others.
What do the numerical results mean?
It’s important to understand the numbers reported on your test result because they help determine how well your treatment is working. These results are an estimate of the amount of leukemia-causing molecules called “BCR-ABL1” in the body. The lower the amount of BCR-ABL1, the less leukemia is present. There are two numbering systems for these test results: %IS and MR.
What is the International Scale? (%IS)
The International Scale is a system to make monitoring BCR-ABL1 lab results more consistent. In general, the lower the number, the less disease is present, and the better your treatment is working. A very low number is the goal of targeted therapy for CML.
In the early days of CML patient monitoring, there was no agreed upon standard way to measure the amount of BCR-ABL1 molecules in the body. This made comparing results from one test to another very difficult. It would be like trying to compare the height of a person in Texas with the height of a person in California—without anyone agreeing on how long a foot or a meter is.
In these early days of CML monitoring, PCR results for BCR-ABL1 were reported as an arbitrary percentage (%) based on how that specific lab calculated results. In order to generate these test results, most labs used different procedures, equipment, and materials. This previously led to a lot of differences between the results given to patients. A value of 20% in one lab might be 0.2% in another lab.
Consistency in results reporting was needed to ensure that a patient’s response to treatment could be reliably monitored, even if results came from different labs. To solve this problem, much like everyone agreeing on the length of a foot, the International Scale (IS) was developed to bring uniformity to BCR-ABL1 results.6 On this new scale, a value of 100% was the average value from a clinical study of an untreated patient with chronic phase CML. An individual’s results could be higher or lower. This is simply an agreed-upon starting place—called a “baseline”—to give everyone in the world a common reference to monitor the disease.
On a lab report, these numbers might be listed as %IS or IS%—or in some cases, simply %.
Your doctor should confirm that BCR-ABL1 results from a lab are being provided on the International Scale. If they’re not, the reported numbers may be inconsistent. Since treatment guidelines require test results to be reported on the International Scale,3 your doctor should require results to be on the IS so they can apply the standard of care for CML.
What are MR values?
MR stands for “Molecular Response”. It is a system for showing how much improvement the patient has achieved since baseline. In other words, MR is a system for demonstrating how many BCR-ABL1 molecules have been eliminated. In general, the higher the number, the better the treatment is working, and the less disease is present.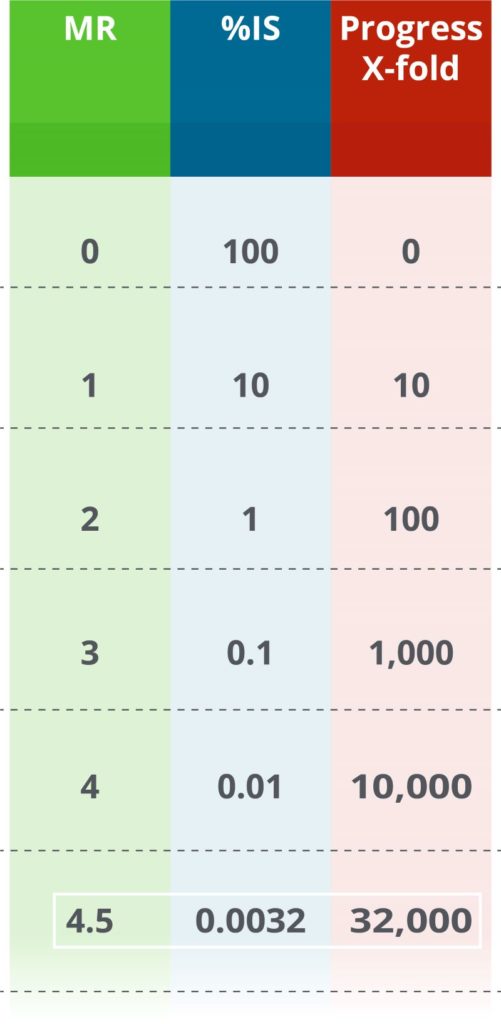
MR is based on the International Scale (%IS values). Every time MR goes up by 1, it means that the leukemia level is 10 times lower. In other words, if an MR value increases by 1, the number of BCR-ABL1 molecules is 10 times lower than the previous measurement. For example, if MR values go from MR2 to MR3, the corresponding %IS values go from 1%IS to 0.1%IS—an indication that disease level has reduced 10x.
Previously, MR values were only reported for a few spots during monitoring, such as MR4 and MR4.5. Some labs still report MR only if the results fit into one of these two levels. For example, these labs will never report MR4.2 (0.0063%), only that MR4 was achieved. Your doctor can help distinguish between these possible scenarios.
Is %IS or MR preferable?
Each numbering scale has pros and cons. It is important to remember that %IS values all have equivalent MR values. How the results are reported is often a matter of preference or historical precedent.
Some people prefer to look at %IS values because those values decrease as the level of leukemia in the body also decreases. However, %IS numbers can get very small and confusing. For example, 0.032%IS and 0.0032%IS look similar, even though they are 10 times different from each other.
Many people think of higher numbers as better. Since a higher MR indicates a better molecular response, some people prefer to review results as MR. It is easier for some people to look at the difference between MR3.5 and MR4.5 (equivalent to 0.032% and 0.0032%, respectively). This difference indicates a 10 times better molecular response than the previous result.
MR values can also make it easier to review meaningful changes. Suppose a result of 0.2483%IS is seen on one visit and then 0.2540%IS from a visit 3 months later. It might look like the numbers went up (worsened), which could be true, or it could be the small natural variability in lab measurements. Either way, it’s not a meaningful difference. It is easier to see how small the change is in this situation when reviewed in the other scale: MR2.61 and MR2.60, respectively. Your doctor will also interpret any changes in numbers in the context of other clinical data.
What is the significance of MR4.5?
MR4.5 is a measure of very low remaining BCR-ABL1 molecules. It’s equivalent to 0.0032%IS, roughly 32,000-fold reduced from diagnosis. In clinical studies so far, none of the CML patients who achieved MR4.5 progressed to advanced phase CML.7,8,9,10 Though there is no cure for CML, MR4.5 is the deepest form of “safe harbor” that can be measured. Importantly, CML treatment guidelines require that the patient have access to a PCR test with a sensitivity of at least MR4.5 if they want to discuss stopping treatment with their doctor.3
Are all PCR monitoring tests the same?
No, not all PCR tests are created equal. Some tests can give inaccurate or highly variable results. This means they give results that are false, or that results change randomly each time the test is performed. Many tests are not cleared by the FDA, meaning they don’t have the benefit of stringent regulatory review for safety and efficacy. Most critically, many tests cannot measure the levels of Deep Molecular Response required for the standard of care, which would make it difficult for you and your doctor to understand if your BCR-ABL1 results are low, lower, or extremely low.
How often is monitoring performed for CML patients?
Monitoring of BCR-ABL1 levels should be performed every 3 months, as recommended by National Comprehensive Cancer Network (NCCN) guidelines. There is an opportunity to relax this a little bit (up to every 6 months) once your results have been below 1%IS (same as greater than MR2) for at least 2 years. However, monitoring frequently helps to catch potential problems earlier.3,12 As an added benefit, multiple studies have shown that, when performed early and as per the recommended schedule, molecular monitoring reduces overall healthcare costs.5,12
These guidelines can change over time, so consulting your doctor is critical.
What about stopping the medication?
Stopping medication without disease progression is possible in certain situations. When TKI medications were first released, these lifesaving drugs were believed to be lifelong therapies for all patients. However, some people may want to attempt to stop taking the medication permanently. Thankfully, some exciting recent developments have allowed some patients to stop taking their TKI medication without their CML progressing.
To clarify, this is not a discussion of a “drug holiday”, which is when someone stops taking a medication temporarily for a particular reason, such as reducing side effects. Choosing to go on a drug holiday without consulting your doctor is not recommended in CML. In fact, not taking the medication as prescribed is a key indicator of poor clinical outcomes.12
Instead, this is a discussion about treatment discontinuation in an attempt to achieve treatment-free remission (TFR). In other words, there is the potential to have low disease activity even without treatment. It is important to know that not everyone is eligible to attempt TFR and not everyone may want to try it. Only you and your doctor can assess together whether stopping treatment is desired and safe to try.
For about half of the people who discontinue their TKI medication, the leukemia comes back within 6 months. However, this is ok since they can start taking the TKI again. The other half of people can go for years with very little evidence of leukemia in their blood. Even if they show detectable levels of BCR-ABL1 (a positive PCR result), it might be safe so long as the number doesn’t go above 0.1%IS (below MR3).10,11 This is why treatment-free remission is sometimes called a “functional cure”. Evidence of CML can still be observed, but the remission is stable even without medication.
What factors influence whether a CML patient can attempt to go treatment free?
Many factors come into play when deciding if someone should attempt to go treatment-free. It is critical to work with your doctor to consider this carefully. Currently, about half of CML patients are deemed eligible to attempt TFR.13
A key factor required for attempting TFR is access to an extremely sensitive lab test. You will also need to get PCR monitoring done more frequently for a while. These two items help ensure that your doctor can see early signs of the disease coming back.3
Below is a quick checklist, which is not exhaustive, of items required for TFR. These guidelines may be updated frequently. Reviewing the opportunity with your doctor is required.
CML patient treatment guidelines from the National Comprehensive Cancer Network have many requirements to attempt TFR,3 including:
- Consultation with a CML specialist
- At least 18 years old
- Chronic phase CML (no history of advanced or blast phase CML)
- Taking TKI for >3 years
- Prior positive result in a BCR-ABL1 PCR test
- Deep Molecular Response: ≤0.01%IS (≥MR4) for ≥2 years (longer time may be better) based on the recommended monitoring schedule
- Access to a PCR test that reports values on the International Scale (%IS and/or MR)
- Access to a PCR test with a sensitivity of at least MR4.5 (0.0032%IS)
- Access to a PCR test with <2 weeks turnaround time
- Willingness for more frequent PCR monitoring:
- Every 1 month during year 1
- Every 2 months during year 2
- Every 3 months indefinitely as long as >MR3 (<0.1%IS)
- If <MR3 (>0.1%IS), resume TKI, then monitor every month until >MR3 (<0.1%IS), and then every 3 months indefinitely
What is the Asuragen QuantideX qPCR BCR-ABL IS Kit?
The QuantideX qPCR BCR-ABL IS Kit is the first FDA-cleared test for monitoring CML. It is the result of 10 years of research and development. This test is used by clinical labs to provide PCR results that help determine how well CML therapy is working in a given patient. The QuantideX kit goes beyond the standard of care for sensitivity (>MR4.5). Importantly, it provides consistently accurate results on the International Scale.
- PCR test
- Works with blood samples
- Reports values on the International Scale (%IS and MR)
- Sensitivity of MR4.7 (0.0020%IS)
- Fast turnaround time
Does the choice of monitoring test matter?
Yes, the best outcomes are supported by the best results. It is important that your CML monitoring results are accurate to provide you and your doctor with the best information for your care.
The QuantideX qPCR BCR-ABL IS Kit uses unique technical innovations that are not included in other assays. One major innovation is the inclusion of multiple calibrators that trace directly to the World Health Organization’s standards for BCR-ABL testing to ensure results are consistently accurate.
To ensure your CML is being safely monitored, accurate BCR-ABL results are required. Ask your doctor if your results are from the QuantideX qPCR BCR-ABL IS Kit.
Glossary
Definitions
- %IS: an estimate of the level of leukemia in the blood, lower numbers mean less evidence of leukemia
- Advanced Phase: a rapidly progressing disease that is Accelerated (rapid uncontrolled growth) and then Blast crisis (difficult to control and dangerous)
- BCR-ABL1: abnormal genetic molecule created by the Philadelphia chromosome that cause CML, sometimes called a “fusion gene”
- Bone Marrow: spongy tissue inside some bones (e.g. hip and thigh) that produces many types of blood cells
- Breakpoints: the exact spot where there is a break in the genes involved in CML
- Chronic Phase: a long, slow-growing period of CML, often without symptoms, but can progress to Advanced Phase if not treated
- Chromosome: a long strand of DNA
- Complete Hematologic Response: CHR, results from blood work have returned to normal, including white blood cells and platelets
- Complete Cytogenetic Response: CCyR, cytogenetic results where the Philadelphia chromosome is undetectable, roughly equivalent to ≤1%IS (≥MR2), 100-fold lower than at diagnosis
- Cytogenetic Test: a method of examining the structure of the chromosomes, can include assessing the presence or amount of the Philadelphia chromosome
- Deep Molecular Response: DMR, a sustained period of monitoring where PCR results for BCR-ABL1 are extremely low (<0.01%IS, >MR4), 10,000-fold lower than at diagnosis
- Early Molecular Response: EMR, PCR results for BCR-ABL1 have reduced to ≤10%IS (≥MR1), 10-fold lower than at diagnosis, at both 3 and 6 months after starting TKI
- Incidence: number of new cases identified for a disease
- International Scale: IS, a standardized scale for estimating the level of leukemic cells in the blood by measuring BCR-ABL1 (reported as %IS)
- Leukemia: a group of cancers that affect blood cells
- Leukemic cells: white blood cells that multiply abnormally; they are immature, meaning that they’re not ready for their normal functions like destroying infectious agents
- Log reduction: a 10-fold drop in BCR-ABL1 results, such as MR1 to MR2
- Major Molecular Response: MMR, PCR results for BCR-ABL1 have reduced to ≤0.1%IS (≥MR3) after starting TKI treatment, 1,000-fold lower than at diagnosis
- Minimal Residual Disease (sometimes called Measurable Residual Disease): MRD, general term for the low number of leukemic cells that might persist after treatment
- Monitoring: a program of testing over time to assess how well CML therapy is working
- MR value: molecular response, an estimate of the level of leukemia in the blood, higher numbers mean less evidence of leukemia
- Myeloid: relating to bone marrow and to red blood cells, platelets, and certain types of white blood cells
- PCR: Polymerase Chain Reaction, a specialized method to estimate the amount of BCR-ABL1 in the blood (also called RT-qPCR or qPCR)
- Philadelphia Chromosome: a random mistake in the DNA that causes CML, sometimes abbreviated Ph+
- Prevalence: how many people are living with the disease
- t(9;22): the translocation also known as the Philadelphia chromosome, resulting from chromosomes 9 and 22 switching sections with each other
- Treatment-Free Remission: TFR, a lifelong period of monitoring where PCR results for BCR-ABL1 are extremely low or undetectable after discontinuing TKI therapy
- Tyrosine Kinase Inhibitor: TKI, a class of medications that target the molecules that cause CML
- Translocation: a type of mistake in DNA where two sections break and then switch locations with each other
- White Blood Cell: WBC, also called a leukocyte, is a cell that circulates in the body and help fight infection and disease
Resources
- ACS: American Cancer Society
- ASCO: American Society of Clinical Oncology
- Cancer Care: Counseling, Support Groups, Education, Financial Assistance
- CML Advocates Network
- iCMLf: International CML Foundation
- LLS: Leukemia and Lymphoma Society
- Max Foundation
- NCCN: National Comprehensive Cancer Network
- NCCS: National Coalition for Cancer Survivorship
- NIH NCI: National Institutes of Health, National Cancer Institute
Citations
- American Cancer Society. (2020, Jan 8). Key Statistics for Chronic Myeloid Leukemia. https://www.cancer.org/cancer/chronic-myeloid-leukemia/about/statistics.html
- Huang, X., et al. (2012). Estimations of the increasing prevalence and plateau prevalence of chronic myeloid leukemia in the era of tyrosine kinase inhibitor therapy. Cancer, 118(12), 3123–3127.
- National Comprehensive Cancer Network. (2020, Jan 30). NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®), Chronic Myeloid Leukemia (version 3.2020). https://www.nccn.org/professionals/physician_gls/pdf/cml.pdf
- Lemonick, M. D. and Park, A. (2001, May 28). New Hope For Cancer. Time, 157(21), cover.
- Jabbour, E. J., et al. (2019). Impact of earlier versus later monitoring on disease progression and healthcare costs among patients with chronic myeloid leukemia in the United States. Leukemia & lymphoma, 60(3), 668–674.
- Hughes, T., et al. (2006). Monitoring CML patients responding to treatment with tyrosine kinase inhibitors: review and recommendations for harmonizing current methodology for detecting BCR-ABL transcripts and kinase domain mutations and for expressing results. Blood, 108(1), 28–37.
- Hehlmann, R., et al. (2014). Deep molecular response is reached by the majority of patients treated with imatinib, predicts survival, and is achieved more quickly by optimized high-dose imatinib: results from the randomized CML-study IV. Journal of clinical oncology: official journal of the American Society of Clinical Oncology, 32(5), 415–423.
- Kantarjian, H. M., et al. (2011). Nilotinib versus imatinib for the treatment of patients with newly diagnosed chronic phase, Philadelphia chromosome-positive, chronic myeloid leukaemia: 24-month minimum follow-up of the phase 3 randomised ENESTnd trial. The Lancet. Oncology, 12(9), 841–851.
- Kantarjian, H. M., et al. (2012). Dasatinib or imatinib in newly diagnosed chronic-phase chronic myeloid leukemia: 2-year follow-up from a randomized phase 3 trial (DASISION). Blood, 119(5), 1123–1129.
- Rousselot, P., et al. (2014). Loss of major molecular response as a trigger for restarting tyrosine kinase inhibitor therapy in patients with chronic-phase chronic myelogenous leukemia who have stopped imatinib after durable undetectable disease. Journal of clinical oncology: official journal of the American Society of Clinical Oncology, 32(5), 424–430.
- Laneuville P. (2018). When to Stop Tyrosine Kinase Inhibitors for the Treatment of Chronic Myeloid Leukemia. Current treatment options in oncology, 19(3), 15.
- Guérin, A., et al. (2014). Association between regular molecular monitoring and tyrosine kinase inhibitor therapy adherence in chronic myelogenous leukemia in the chronic phase. Current medical research and opinion, 30(7), 1345–1352.
- Mauro, M. (2019, Feb). Understanding Treatment-Free Remission and How It Impacts You: Frequently Asked Questions for Patients and Advocates. The Max Foundation. http://mypcr.org/wp-content/uploads/2019/02/My_PCR_TFR_FAQ.pdf
CML Patients Deserve Better Answers
Welcome
A cancer diagnosis is scary, but today, with treatment and careful monitoring, most patients living with chronic myeloid leukemia (CML) have near-normal life expectancies. CML is manageable thanks to advances in testing and treatment options.3
This site is designed to give CML patients and their loved ones the resources to feel empowered and get the best answers they can. The terminology is based on guidelines in the United States. Variations exist in other regions, and guidelines change over time, so it’s critical to consult with a doctor for any questions or concerns you may have.
CML Overview
What is Chronic Myeloid Leukemia (CML)?
Chronic Myeloid Leukemia (CML) is a type of cancer that starts in the bone marrow. It results in immature white blood cells growing out of control. This guide covers the chronic phase of CML, which is when most patients are diagnosed.
What causes CML?
CML comes from a random mutation in genes. It’s not likely that you did anything that led to this disease. It is more common as people age, and it’s slightly more common in men than in women. In rare instances, it can come from being exposed to high doses of radiation. Otherwise, no other risk factors are known.
CML is not passed down through family genes. It also can’t be passed around like a cold, so it wasn’t caught from anyone else.
It’s simply a random event that causes CML.
How common is CML?
In the US, there are more than 8,000 new cases of CML diagnosed every year. It accounts for about 15% of all types of leukemia (blood cancers) in adults. It can happen at any age, but the average age at diagnosis is about 64.1
The number of people living with CML has increased over the years. This is partly good news because it means people with CML are living longer due to advances in treatment and monitoring. In the US, it is estimated that there will be at least 140,000 people living with CML by 2030.2
How is CML diagnosed?
CML is usually suspected based on routine blood work, which is one reason why it’s important to have an annual checkup. A few people might experience some symptoms, like fatigue or night sweats, but most don’t.
If CML is suspected on routine bloodwork, it will need to be confirmed with multiple tests requiring blood and bone marrow samples. An important part of the diagnosis is identifying the presence of the “Philadelphia chromosome”, which everyone with CML has. The Philadelphia chromosome is formed when your DNA randomly breaks, and your body puts it back together improperly. Your clinician will also need to determine the point at which the DNA broke, sometimes called a “breakpoint”. This guide is written about the specific breakpoint that about 99% of CML patients have—hence, it’s called the “Major” breakpoint.
Once CML is confirmed, it’s extremely important to get treatment and monitoring—even if you don’t feel sick. The excess growth of cancer cells eventually crowds out healthy cells, which can lead to severe sickness and death.3
How is CML treated?
Targeted medications are available. CML is typically treated with a class of medication called tyrosine kinase inhibitors (TKIs).3 The first TKI on the market is called Gleevec® (Novartis), or by its generic name imatinib. It was featured on the cover of Time magazine in 2001 because it was such a revolutionary breakthrough for CML treatment.4 Since then, additional TKIs have been approved. You will need to work with your doctor to determine the best medication and dosage specifically for you.
CML Monitoring
Why do CML patients need monitoring?
Monitoring is critical since it’s a key part of how most CML cases were transformed from life-threatening to manageable. The purpose of monitoring is to determine how well the medication is doing its job. Monitoring provides you with reassurance that the disease is responding to treatment, and a good response may predict a good outcome over the years.
As a CML patient, if you do not monitor your disease, you run the risk of failing to see that the disease might not be responding to treatment. Or you might fail to see that the disease had been responding to treatment and then quit responding—which could be a sign of relapse. This can be due to different underlying causes, such as developing resistance. Thankfully, if your medication isn’t working, your physician may be able to switch you to a more effective treatment. For all of these important reasons, monitoring is essential to tell how your current treatment is working. The good news is that most monitoring today only requires a visit to the doctor and a blood sample.3
In addition, studies have shown that monitoring early and on the recommended schedule reduces overall healthcare costs.5,12
How is CML monitoring done?
 Monitoring uses multiple lab tests.3 Initially, general blood work could give a rough idea of response to treatment. Since CML was likely suspected based on a high white blood cell count, seeing it return to a normal level is a good start—but it’s not enough (see “CHR”). Next, tests might be run to look for the Philadelphia chromosome itself which should disappear over the course of treatment (see “CCyR”). Though this is an even better sign, it’s still not sufficient to be sure that the leukemia is at low enough levels in your body.
Monitoring uses multiple lab tests.3 Initially, general blood work could give a rough idea of response to treatment. Since CML was likely suspected based on a high white blood cell count, seeing it return to a normal level is a good start—but it’s not enough (see “CHR”). Next, tests might be run to look for the Philadelphia chromosome itself which should disappear over the course of treatment (see “CCyR”). Though this is an even better sign, it’s still not sufficient to be sure that the leukemia is at low enough levels in your body.
In order to measure very small amounts of leukemia molecules that are in the body, molecular methods (e.g. PCR, qPCR, and RT-qPCR) are used by many laboratories. PCR can be a very sensitive and accurate laboratory method used to estimate the amount of leukemia molecules, called “BCR-ABL1”, in the body. These molecules come from the Philadelphia chromosome. So, the lower the amount of BCR-ABL1, the less leukemia is present. The good news is that the PCR test is performed with a blood sample.
PCR was initially used for CML because of its potential to detect disease at very low levels. Once the blood cell counts (CHR) and chromosome counts (CCyR) were back to normal, monitoring by PCR would begin. However, now it’s important to be sure that PCR is ordered from the very start of your diagnosis and throughout lifelong monitoring. This is because your doctor needs to be certain that you have the common form of BCR-ABL1 that can be monitored. Rare forms exist that cannot be monitored by typical PCR tests.3
Finally, be aware that not all PCR tests are created equal. Some may give more consistent, accurate results than others.
What do the numerical results mean?
It’s important to understand the numbers reported on your test result because they help determine how well your treatment is working. These results are an estimate of the amount of leukemia-causing molecules called “BCR-ABL1” in the body. The lower the amount of BCR-ABL1, the less leukemia is present. There are two numbering systems for these test results: %IS and MR.
What is the International Scale? (%IS)
The International Scale is a system to make monitoring BCR-ABL1 lab results more consistent. In general, the lower the number, the less disease is present, and the better your treatment is working. A very low number is the goal of targeted therapy for CML.
In the early days of CML patient monitoring, there was no agreed upon standard way to measure the amount of BCR-ABL1 molecules in the body. This made comparing results from one test to another very difficult. It would be like trying to compare the height of a person in Texas with the height of a person in California—without anyone agreeing on how long a foot or a meter is.
In these early days of CML monitoring, PCR results for BCR-ABL1 were reported as an arbitrary percentage (%) based on how that specific lab calculated results. In order to generate these test results, most labs used different procedures, equipment, and materials. This previously led to a lot of differences between the results given to patients. A value of 20% in one lab might be 0.2% in another lab.
Consistency in results reporting was needed to ensure that a patient’s response to treatment could be reliably monitored, even if results came from different labs. To solve this problem, much like everyone agreeing on the length of a foot, the International Scale (IS) was developed to bring uniformity to BCR-ABL1 results.6 On this new scale, a value of 100% was the average value from a clinical study of an untreated patient with chronic phase CML. An individual’s results could be higher or lower. This is simply an agreed-upon starting place—called a “baseline”—to give everyone in the world a common reference to monitor the disease.
On a lab report, these numbers might be listed as %IS or IS%—or in some cases, simply %.
Your doctor should confirm that BCR-ABL1 results from a lab are being provided on the International Scale. If they’re not, the reported numbers may be inconsistent. Since treatment guidelines require test results to be reported on the International Scale,3 your doctor should require results to be on the IS so they can apply the standard of care for CML.
What are MR values?
MR stands for “Molecular Response”. It is a system for showing how much improvement the patient has achieved since baseline. In other words, MR is a system for demonstrating how many BCR-ABL1 molecules have been eliminated. In general, the higher the number, the better the treatment is working, and the less disease is present.
MR is based on the International Scale (%IS values). Every time MR goes up by 1, it means that the leukemia level is 10 times lower. In other words, if an MR value increases by 1, the number of BCR-ABL1 molecules is 10 times lower than the previous measurement. For example, if MR values go from MR2 to MR3, the corresponding %IS values go from 1%IS to 0.1%IS—an indication that disease level has reduced 10x.
Previously, MR values were only reported for a few spots during monitoring, such as MR4 and MR4.5. Some labs still report MR only if the results fit into one of these two levels. For example, these labs will never report MR4.2 (0.0063%), only that MR4 was achieved. Your doctor can help distinguish between these possible scenarios.
Is %IS or MR preferable?
Each numbering scale has pros and cons. It is important to remember that %IS values all have equivalent MR values. How the results are reported is often a matter of preference or historical precedent.
Some people prefer to look at %IS values because those values decrease as the level of leukemia in the body also decreases. However, %IS numbers can get very small and confusing. For example, 0.032%IS and 0.0032%IS look similar, even though they are 10 times different from each other.
Many people think of higher numbers as better. Since a higher MR indicates a better molecular response, some people prefer to review results as MR. It is easier for some people to look at the difference between MR3.5 and MR4.5 (equivalent to 0.032% and 0.0032%, respectively). This difference indicates a 10 times better molecular response than the previous result.
MR values can also make it easier to review meaningful changes. Suppose a result of 0.2483%IS is seen on one visit and then 0.2540%IS from a visit 3 months later. It might look like the numbers went up (worsened), which could be true, or it could be the small natural variability in lab measurements. Either way, it’s not a meaningful difference. It is easier to see how small the change is in this situation when reviewed in the other scale: MR2.61 and MR2.60, respectively. Your doctor will also interpret any changes in numbers in the context of other clinical data.
What is the significance of MR4.5?
MR4.5 is a measure of very low remaining BCR-ABL1 molecules. It’s equivalent to 0.0032%IS, roughly 32,000-fold reduced from diagnosis. In clinical studies so far, none of the CML patients who achieved MR4.5 progressed to advanced phase CML.7,8,9,10 Though there is no cure for CML, MR4.5 is the deepest form of “safe harbor” that can be measured. Importantly, CML treatment guidelines require that the patient have access to a PCR test with a sensitivity of at least MR4.5 if they want to discuss stopping treatment with their doctor.3
Are all PCR monitoring tests the same?
No, not all PCR tests are created equal. Some tests can give inaccurate or highly variable results. This means they give results that are false, or that results change randomly each time the test is performed. Many tests are not cleared by the FDA, meaning they don’t have the benefit of stringent regulatory review for safety and efficacy. Most critically, many tests cannot measure the levels of Deep Molecular Response required for the standard of care, which would make it difficult for you and your doctor to understand if your BCR-ABL1 results are low, lower, or extremely low.
How often is monitoring performed for CML patients?
Monitoring of BCR-ABL1 levels should be performed every 3 months, as recommended by National Comprehensive Cancer Network (NCCN) guidelines. There is an opportunity to relax this a little bit (up to every 6 months) once your results have been below 1%IS (same as greater than MR2) for at least 2 years. However, monitoring frequently helps to catch potential problems earlier.3,12 As an added benefit, multiple studies have shown that, when performed early and as per the recommended schedule, molecular monitoring reduces overall healthcare costs.5,12
These guidelines can change over time, so consulting your doctor is critical.
What about stopping the medication?
Stopping medication without disease progression is possible in certain situations. When TKI medications were first released, these lifesaving drugs were believed to be lifelong therapies for all patients. However, some people may want to attempt to stop taking the medication permanently. Thankfully, some exciting recent developments have allowed some patients to stop taking their TKI medication without their CML progressing.
To clarify, this is not a discussion of a “drug holiday”, which is when someone stops taking a medication temporarily for a particular reason, such as reducing side effects. Choosing to go on a drug holiday without consulting your doctor is not recommended in CML. In fact, not taking the medication as prescribed is a key indicator of poor clinical outcomes.12
Instead, this is a discussion about treatment discontinuation in an attempt to achieve treatment-free remission (TFR). In other words, there is the potential to have low disease activity even without treatment. It is important to know that not everyone is eligible to attempt TFR and not everyone may want to try it. Only you and your doctor can assess together whether stopping treatment is desired and safe to try.
For about half of the people who discontinue their TKI medication, the leukemia comes back within 6 months. However, this is ok since they can start taking the TKI again. The other half of people can go for years with very little evidence of leukemia in their blood. Even if they show detectable levels of BCR-ABL1 (a positive PCR result), it might be safe so long as the number doesn’t go above 0.1%IS (below MR3).10,11 This is why treatment-free remission is sometimes called a “functional cure”. Evidence of CML can still be observed, but the remission is stable even without medication.
What factors influence whether a CML patient can attempt to go treatment free?
Many factors come into play when deciding if someone should attempt to go treatment-free. It is critical to work with your doctor to consider this carefully. Currently, about half of CML patients are deemed eligible to attempt TFR.13
A key factor required for attempting TFR is access to an extremely sensitive lab test. You will also need to get PCR monitoring done more frequently for a while. These two items help ensure that your doctor can see early signs of the disease coming back.3
Below is a quick checklist, which is not exhaustive, of items required for TFR. These guidelines may be updated frequently. Reviewing the opportunity with your doctor is required.
CML patient treatment guidelines from the National Comprehensive Cancer Network have many requirements to attempt TFR,3 including:
- Consultation with a CML specialist
- At least 18 years old
- Chronic phase CML (no history of advanced or blast phase CML)
- Taking TKI for >3 years
- Prior positive result in a BCR-ABL1 PCR test
- Deep Molecular Response: ≤0.01%IS (≥MR4) for ≥2 years (longer time may be better) based on the recommended monitoring schedule
- Access to a PCR test that reports values on the International Scale (%IS and/or MR)
- Access to a PCR test with a sensitivity of at least MR4.5 (0.0032%IS)
- Access to a PCR test with <2 weeks turnaround time
- Willingness for more frequent PCR monitoring:
- Every 1 month during year 1
- Every 2 months during year 2
- Every 3 months indefinitely as long as >MR3 (<0.1%IS)
- If <MR3 (>0.1%IS), resume TKI, then monitor every month until >MR3 (<0.1%IS), and then every 3 months indefinitely
What is the Asuragen QuantideX qPCR BCR-ABL IS Kit?
The QuantideX qPCR BCR-ABL IS Kit is the first FDA-cleared test for monitoring CML. It is the result of 10 years of research and development. This test is used by clinical labs to provide PCR results that help determine how well CML therapy is working in a given patient. The QuantideX kit goes beyond the standard of care for sensitivity (>MR4.5). Importantly, it provides consistently accurate results on the International Scale.
- PCR test
- Works with blood samples
- Reports values on the International Scale (%IS and MR)
- Sensitivity of MR4.7 (0.0020%IS)
- Fast turnaround time
Does the choice of monitoring test matter?
Yes, the best outcomes are supported by the best results. It is important that your CML monitoring results are accurate to provide you and your doctor with the best information for your care.
The QuantideX qPCR BCR-ABL IS Kit uses unique technical innovations that are not included in other assays. One major innovation is the inclusion of multiple calibrators that trace directly to the World Health Organization’s standards for BCR-ABL testing to ensure results are consistently accurate.
To ensure your CML is being safely monitored, accurate BCR-ABL results are required. Ask your doctor if your results are from the QuantideX qPCR BCR-ABL IS Kit.
Glossary
Definitions
- %IS: an estimate of the level of leukemia in the blood, lower numbers mean less evidence of leukemia
- Advanced Phase: a rapidly progressing disease that is Accelerated (rapid uncontrolled growth) and then Blast crisis (difficult to control and dangerous)
- BCR-ABL1: abnormal genetic molecule created by the Philadelphia chromosome that cause CML, sometimes called a “fusion gene”
- Bone Marrow: spongy tissue inside some bones (e.g. hip and thigh) that produces many types of blood cells
- Breakpoints: the exact spot where there is a break in the genes involved in CML
- Chronic Phase: a long, slow-growing period of CML, often without symptoms, but can progress to Advanced Phase if not treated
- Chromosome: a long strand of DNA
- Complete Hematologic Response: CHR, results from blood work have returned to normal, including white blood cells and platelets
- Complete Cytogenetic Response: CCyR, cytogenetic results where the Philadelphia chromosome is undetectable, roughly equivalent to ≤1%IS (≥MR2), 100-fold lower than at diagnosis
- Cytogenetic Test: a method of examining the structure of the chromosomes, can include assessing the presence or amount of the Philadelphia chromosome
- Deep Molecular Response: DMR, a sustained period of monitoring where PCR results for BCR-ABL1 are extremely low (<0.01%IS, >MR4), 10,000-fold lower than at diagnosis
- Early Molecular Response: EMR, PCR results for BCR-ABL1 have reduced to ≤10%IS (≥MR1), 10-fold lower than at diagnosis, at both 3 and 6 months after starting TKI
- Incidence: number of new cases identified for a disease
- International Scale: IS, a standardized scale for estimating the level of leukemic cells in the blood by measuring BCR-ABL1 (reported as %IS)
- Leukemia: a group of cancers that affect blood cells
- Leukemic cells: white blood cells that multiply abnormally; they are immature, meaning that they’re not ready for their normal functions like destroying infectious agents
- Log reduction: a 10-fold drop in BCR-ABL1 results, such as MR1 to MR2
- Major Molecular Response: MMR, PCR results for BCR-ABL1 have reduced to ≤0.1%IS (≥MR3) after starting TKI treatment, 1,000-fold lower than at diagnosis
- Minimal Residual Disease (sometimes called Measurable Residual Disease): MRD, general term for the low number of leukemic cells that might persist after treatment
- Monitoring: a program of testing over time to assess how well CML therapy is working
- MR value: molecular response, an estimate of the level of leukemia in the blood, higher numbers mean less evidence of leukemia
- Myeloid: relating to bone marrow and to red blood cells, platelets, and certain types of white blood cells
- PCR: Polymerase Chain Reaction, a specialized method to estimate the amount of BCR-ABL1 in the blood (also called RT-qPCR or qPCR)
- Philadelphia Chromosome: a random mistake in the DNA that causes CML, sometimes abbreviated Ph+
- Prevalence: how many people are living with the disease
- t(9;22): the translocation also known as the Philadelphia chromosome, resulting from chromosomes 9 and 22 switching sections with each other
- Treatment-Free Remission: TFR, a lifelong period of monitoring where PCR results for BCR-ABL1 are extremely low or undetectable after discontinuing TKI therapy
- Tyrosine Kinase Inhibitor: TKI, a class of medications that target the molecules that cause CML
- Translocation: a type of mistake in DNA where two sections break and then switch locations with each other
- White Blood Cell: WBC, also called a leukocyte, is a cell that circulates in the body and help fight infection and disease